Professional Documents
Culture Documents
55 PDF
Uploaded by
Camelia Moise0 ratings0% found this document useful (0 votes)
8 views1 pageOriginal Title
55.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
8 views1 page55 PDF
Uploaded by
Camelia MoiseCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
Basic Chemistry of Polystyrene
55
<£
UJ
100,000 200,000 300,000
MOLECULAR WEIGHT
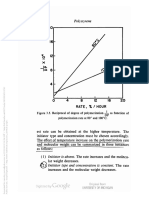
Figure 3.8. Typical molecular weight distribution curve of polystyrene.
[From Schulz and Dinglinger, Z. physik. Chem. 43B, 47 (1939).]
merization is shown in Figure 3.8, where the weight fraction
of polystyrene of a particular molecular weight is plotted as
a function of molecular weight. A sample of polystyrene can be
separated into fractions by fractional precipitation, a process
based on the principle Jhat the solubility of polystyrene de-
crealesIwEer^^
amount of a nonsolvent such as met&nol is added to a solu-
tion of polystyrene, the highest molecular weight material_pre-
cipitates first. This is separated, more methanol is added2and
the next fraction, of lower molecular weight, precipitates. The
process is repeated until a large number of fractions is obtained.,
Number average molecular weights can be measured by
osmotic pressure methods; weight averages can be measured
by light-scattering methods. Since these methods are time
Generated on 2013-04-10 17:09 GMT / http://hdl.handle.net/2027/mdp.39015016511761
Public Domain, Google-digitized / http://www.hathitrust.org/access_use#pd-google
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- 40 PDFDocument1 page40 PDFCamelia MoiseNo ratings yet
- 50 PDFDocument1 page50 PDFCamelia MoiseNo ratings yet
- DATA Space ReplaimDocument3 pagesDATA Space ReplaimCamelia MoiseNo ratings yet
- 44 PDFDocument1 page44 PDFCamelia MoiseNo ratings yet
- 56 PDFDocument1 page56 PDFCamelia MoiseNo ratings yet
- 54 PDFDocument1 page54 PDFCamelia MoiseNo ratings yet
- 42 PDFDocument1 page42 PDFCamelia MoiseNo ratings yet
- 43 PDFDocument1 page43 PDFCamelia MoiseNo ratings yet
- 41 PDFDocument1 page41 PDFCamelia MoiseNo ratings yet
- 49 PDFDocument1 page49 PDFCamelia MoiseNo ratings yet
- 51 PDFDocument1 page51 PDFCamelia MoiseNo ratings yet
- 47 PDFDocument1 page47 PDFCamelia MoiseNo ratings yet
- 46 PDFDocument1 page46 PDFCamelia MoiseNo ratings yet
- 52 PDFDocument1 page52 PDFCamelia MoiseNo ratings yet
- 48 PDFDocument1 page48 PDFCamelia MoiseNo ratings yet
- 53 PDFDocument1 page53 PDFCamelia MoiseNo ratings yet
- 58 PDFDocument1 page58 PDFCamelia MoiseNo ratings yet
- 57 PDFDocument1 page57 PDFCamelia MoiseNo ratings yet
- 59 PDFDocument1 page59 PDFCamelia MoiseNo ratings yet
- Polystyrene Production via Suspension PolymerizationDocument1 pagePolystyrene Production via Suspension PolymerizationCamelia MoiseNo ratings yet
- 45 PDFDocument1 page45 PDFCamelia MoiseNo ratings yet
- 60 PDFDocument1 page60 PDFCamelia MoiseNo ratings yet
- 65 PDFDocument1 page65 PDFCamelia MoiseNo ratings yet
- Manufacture of Polystyrene Process DetailsDocument1 pageManufacture of Polystyrene Process DetailsCamelia MoiseNo ratings yet
- 64 PDFDocument1 page64 PDFCamelia MoiseNo ratings yet
- 62 PDFDocument1 page62 PDFCamelia MoiseNo ratings yet
- 102 PDFDocument1 page102 PDFCamelia MoiseNo ratings yet
- 104 PDFDocument1 page104 PDFCamelia MoiseNo ratings yet
- 99 PDFDocument1 page99 PDFCamelia MoiseNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)