Professional Documents
Culture Documents
QSP 01 - Document Control Procedure
Uploaded by
Vivek VCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
QSP 01 - Document Control Procedure
Uploaded by
Vivek VCopyright:
Available Formats
QuEST GLOBAL MANUFACTURING PRIVATE LIMITED
UNIT-III
QUALITY SYSTEM PROCEDURE
[ISO 9001: 2008, ISO/TS 29001: 2010 & API SPEC Q1, 8TH EDITION]
DOCUMENT CONTROL PROCEDURE
AMENDMENT HISTORY
Issue Issue /Rev.
Sl. No. Description of the change
/Rev No. Date
A New System Procedure developed as per the ISO 9001:
1 01/00 02 Sep 2013/- 2008, API Spec Q1 8th Edition and ISO TS 29001: 2010
requirements.
1.0 PURPOSE
To define the methodology followed by QuEST GLOBAL MANUFACTURING PRIVATE LIMITED for coding,
review, approval, issue, control and change in documents, data. This includes internal and external
documents.
2.0 SCOPE
This procedure is applicable to all the levels of documents pertaining to ISO 9001: 2008, ISO / TS 29001:
2010, API Spec Q1, 8thedition.
All four levels of documents are under the scope of this procedure
This procedure applies to the following external documents:
1. Customer drawings and specifications
2. Customer standards
3. ISO Standards / API Standards
4. And other related standards.
3.0 RESPONSIBILITY
1. Management representative
2. All concerned process heads
4.0 DESCRIPTION
Prepared by Management Representative
Date 02 Sep 2013 Edition No. 01 Rev. No. 00 Page No.
Signature
Approved by Vice President O&G
Date 02 Sep 2013 Date 02 Sep 2013 Date -- 1 of 7
Signature
QuEST GLOBAL MANUFACTURING PRIVATE LIMITED
UNIT-III
QUALITY SYSTEM PROCEDURE
[ISO 9001: 2008, ISO/TS 29001: 2010 & API SPEC Q1, 8TH EDITION]
DOCUMENT CONTROL PROCEDURE
‘QuEST GLOBAL MANUFACTURING PRIVATE LIMITED’ Quality Management System documentation
comprises of the following types of documents:
1. Quality Management System Manual
2. A documented Quality Policy statement (Refer : Quality Manual Annex 08)
3. Documented statements of quality objectives
4. Quality Management System Procedures
5. Work Instructions & VSOP
6. Engineering documents, including drawings, specifications, procedures etc.,
Including coding, review, approval, issue, and control and changing documents and data procedures.
5.0 KEY PROCESS INDICATORS
1.0 Status of Document Change Request Form (ECR Register)
6.0 RECORDS
Description of the Retention Retention Indexing
Sl. No. Format Number
record Period Responsibility Method
01 Document Change O&G / CM / F 01 5 Years Sr. Manager Computer/
Request Form Engineering File
02 Document Issue O&G / CM / F 02 5 Years Sr. Manager Computer/
Control Register Engineering File
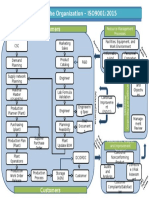
7.0 PROCESS FLOW CHART:
Prepared by Management Representative
Date 02 Sep 2013 Edition No. 01 Rev. No. 00 Page No.
Signature
Approved by Vice President O&G
Date 02 Sep 2013 Date 02 Sep 2013 Date -- 2 of 7
Signature
QuEST GLOBAL MANUFACTURING PRIVATE LIMITED
UNIT-III
QUALITY SYSTEM PROCEDURE
[ISO 9001: 2008, ISO/TS 29001: 2010 & API SPEC Q1, 8TH EDITION]
DOCUMENT CONTROL PROCEDURE
CEO & Chairman
Vice President
Management
Representative
Process Heads
8.0 PROCEDURE:
8.1 PROCEDURE FOR CODING:
8.1.1 Procedure for Coding: - Quality Management System Manual
The sections of Quality Management System manual coded as Section - XX, where XX represents
the running serial number for individual sections. Revision level is identified with running serial
number.
8.1.2 Procedure for Coding: Quality Management System Procedure - Procedures
The sections of Quality Management System Procedures are coded as QSP - XX, Where XX
represents the running serial number for process wise procedures. Revision level is identified with
running serial number.
8.1.3 Procedure for Coding Documents:
All Documents are coded with O&G / XXX / DYY
XXX –Represents the department code
Prepared by Management Representative
Date 02 Sep 2013 Edition No. 01 Rev. No. 00 Page No.
Signature
Approved by Vice President O&G
Date 02 Sep 2013 Date 02 Sep 2013 Date -- 3 of 7
Signature
QuEST GLOBAL MANUFACTURING PRIVATE LIMITED
UNIT-III
QUALITY SYSTEM PROCEDURE
[ISO 9001: 2008, ISO/TS 29001: 2010 & API SPEC Q1, 8TH EDITION]
DOCUMENT CONTROL PROCEDURE
D – Represents the document
YY– Serial Number,
8.1.4 Procedure for Coding Formats
All Formats are coded with O&G / XXX / YY
XXX –Represents the department code
YY– Serial Number,
8.2 Documents Change / Revision
8.2.1 If there is a need for document revision, concerned process raises Engineering Change
Request Form, describing the changes required with reasoning and forwards the same to
MR and Configuration Department.
8.2.2 MR assigns a serial number for the request; review the revision requirements and on
acceptance, forward to approval authority. In case of a change required, the request is
returned.
8.2.3 Approval authority reviews the request and analyses with inputs from MR & also considering
the impact on rest of the system.
8.2.4 Approval of the Engineering change request form and issue to MR.
8.2.5 Communicate reason for denial to concern.
8.2.6 MR incorporates the revision changes in the relevant document with updated revision status,
also updates& revises in the Master List of Documents.
9.0 MAINTENANCE OF SUPERSEDED DOCUMENTS
1. QuEST GLOBAL MANUFACTURING PRIVATE LIMITED manual for Management system is the Quality
Management System Manual. The QMS Manual is available in SOFT. Responsibility of updating Quality
Prepared by Management Representative
Date 02 Sep 2013 Edition No. 01 Rev. No. 00 Page No.
Signature
Approved by Vice President O&G
Date 02 Sep 2013 Date 02 Sep 2013 Date -- 4 of 7
Signature
QuEST GLOBAL MANUFACTURING PRIVATE LIMITED
UNIT-III
QUALITY SYSTEM PROCEDURE
[ISO 9001: 2008, ISO/TS 29001: 2010 & API SPEC Q1, 8TH EDITION]
DOCUMENT CONTROL PROCEDURE
Management System Manual lies with the Management Representative.
2. Quality Management System Procedures are linked to document control system in QMS Manual.
3. QMS Procedures are mapped as per defined processes in QMS Manual.
4. All internal procedures are reviewed and approved by respective process owners and Management
Representative reviews and updates them in hard and soft.
5. Management Representative reviews the procedures for its adequacy in line with ISO 9001: 2008 and
ISO / TS 29001: 2010 / API Spec Q1, 8 th edition, gets it approved from the top authority in the
organization and prints and issues them as controlled copies.
6. If hard copy is required to be kept then procedure has to be released by MR by putting “Controlled Copy”
in Blue color stamp. Distribution is recorded for future updating of the procedure by the Management
Representative.
7. The Management Representative takes the data backup of procedures to prevent the loss of data due to
system failure.
8. Work Instructions / VSOP are reviewed and approved by process owners and process owners have the
responsibility to maintain the master list of work instruction/VSOP and change updating.
9. The Level 01 – Quality Management System Manual (QMSM) is available with the MR with a copy made
accessible to all process owners.
10. The Level 02 – Quality Management System Procedure (QMSP) is made available to all process owners
and is available with them.
11. The Level 03 – Work instructions/ Operation Plan/ Quality Plan / Visual Standard Operating Procedures
are made available to all process personnel / operating personnel and are available at their working point
or point of use.
12. The Level 04 – Records and formats are available with all the process owners and operating personnel
and made available at the point of use.
13. If hard copy of VSOP / work instruction needs to be displayed, then process owner will take a copy and
gets it authorized by MR. MR will check with reference to Master list of documents and stamp the Work
Instruction/VSOP each page with “Controlled Copy” stamp in blue color.
14. Formats are generated by Management Representative and Master List of Records are maintained by
the Management Representative indicating the Format number and its Revision status.
15. All documents have an issue status and issue date on the document identifying the current issue /
revision and dates.
16. Master list is updated to indicate the new issue status and the issue date.
17. If the copies of the customer / internal drawings are lost or damaged by the user departments, the
Prepared by Management Representative
Date 02 Sep 2013 Edition No. 01 Rev. No. 00 Page No.
Signature
Approved by Vice President O&G
Date 02 Sep 2013 Date 02 Sep 2013 Date -- 5 of 7
Signature
QuEST GLOBAL MANUFACTURING PRIVATE LIMITED
UNIT-III
QUALITY SYSTEM PROCEDURE
[ISO 9001: 2008, ISO/TS 29001: 2010 & API SPEC Q1, 8TH EDITION]
DOCUMENT CONTROL PROCEDURE
department head makes a request to the Manager - Engineering for issue of fresh copy of the drawings.
18. The Engineering Department ensures that the old / damaged drawings are made obsolete.
19. All other data stored in the computer is protected with a user password and is accessible only to
respective departments.
20. A system backup is taken by MR on a monthly basis for Internal and external documents stored as soft
copy.
21. QuEST GLOBAL MANUFACTURING PRIVATE LIMITED has transformed from paper to electronic
documentation. All new categories of documents are transferred from paper to electronic document
control system. Both systems are currently used, and are defined in this Control Feature.
22. New documents and document changes may be initiated by anyone in the organization, but may only be
issued by an authorized function. The authorized functions and the rules governing the issue of
documents are defined in this procedure. All documents are reviewed and approved prior to issue.
23. A paper document is officially issued for use when it is approved by authorized function. An electronic
document is issued by being placed in a public directory accessible from the network.
24. Documents are distributed to personnel and locations where they are used. When appropriate and
relevant, documents display a distribution list. Document placements are regulated by this procedure
25. A master list of external origin documents is maintained which is essential for the planning and operation
of the Quality Management System. The master list of external origin document is identified, controlled
and updated as and when changes are made to the standard.
26. Obsolete documents are removed from points of use. Retained masters or copies of obsolete documents
are properly marked with a blue stamp as “OBSOLETE” and are kept separate from active documents.
27. Revised documents are distributed with a change brief summarizing the changes. Controlled documents
will be updated and maintained in the master list specifying the latest issues and revisions of its
documents.
10.0 DOCUMENT CONTROLS
Description of the
Sl. No. User Control
document
01. Quality Management All Process owners & With MR &Top Management /
System Manual applicable users Hardcopy(1 no.) / Softcopy(Read
Prepared by Management Representative
Date 02 Sep 2013 Edition No. 01 Rev. No. 00 Page No.
Signature
Approved by Vice President O&G
Date 02 Sep 2013 Date 02 Sep 2013 Date -- 6 of 7
Signature
QuEST GLOBAL MANUFACTURING PRIVATE LIMITED
UNIT-III
QUALITY SYSTEM PROCEDURE
[ISO 9001: 2008, ISO/TS 29001: 2010 & API SPEC Q1, 8TH EDITION]
DOCUMENT CONTROL PROCEDURE
Only)
02. Quality Management All Process owners & With MR &Top Management /
System Procedures applicable users Hardcopy(1 no.) / Softcopy(Read
Only)
03. Work Instructions / Visual All Process owners & With MR / Top Management /
Standard Operating applicable users Hardcopy / Softcopy
procedures
11.0APPROVAL & RE-APPROVAL AUTHORITY
Level 01 QMS Manual Vice President O&G
Level 02 QMS Procedures Vice President O&G
Level 03 VSOP / Work instruction Management Representative
Level 03 Quality Plan Assistant Manager QA / Manager Projects
Level 03 Skill Matrix Process Owner
Level 04 Master list of Records Management Representative
12.0 API - QMS SPECIFIC REQUIREMENTS
1. QuEST GLOBAL MANUFACTURING PRIVATE LIMITED has established a documented control
feature for control of documents as addressed in QMS Manual Level - 01.
2. Master list of quality system documents is also available to identify & control documentation to
ensure that right information is being communicated to the right people. This also enables that
the current revision status is identified & traceable.
3. The review and approval of the changes is done by the same function (or the same / process /
department), which did the original review and approval.
Prepared by Management Representative
Date 02 Sep 2013 Edition No. 01 Rev. No. 00 Page No.
Signature
Approved by Vice President O&G
Date 02 Sep 2013 Date 02 Sep 2013 Date -- 7 of 7
Signature
You might also like
- qp-001 Rev5 2011Document13 pagesqp-001 Rev5 2011api-177803962No ratings yet
- Internal Quality Audit Plan Dilg Region 10Document8 pagesInternal Quality Audit Plan Dilg Region 10Cess AyomaNo ratings yet
- ISO 9001:2008 Standard Operating Procedures Manual: A P & C, IDocument88 pagesISO 9001:2008 Standard Operating Procedures Manual: A P & C, IBuenoflor GrandeaNo ratings yet
- SOP-01 (Procedure For Document Control)Document8 pagesSOP-01 (Procedure For Document Control)FarhanNo ratings yet
- P018 Internal Audit Procedure: ISO 9001:2008 Clause 8.2.2Document9 pagesP018 Internal Audit Procedure: ISO 9001:2008 Clause 8.2.2Álvaro Martínez Fernández100% (1)
- External Origin Documents ListDocument2 pagesExternal Origin Documents ListSyed Mujtaba Ali Bukhari100% (1)
- Procedure For Control of RecordsDocument3 pagesProcedure For Control of Recordsmatrixmaze50% (2)
- Procedure of Document ControlDocument5 pagesProcedure of Document ControlNguyễn Văn GiápNo ratings yet
- Document Control Procedure ExampleDocument4 pagesDocument Control Procedure ExampleTofiq Hussein33% (3)
- Implementing an Integrated Management System (IMS): The strategic approachFrom EverandImplementing an Integrated Management System (IMS): The strategic approachRating: 5 out of 5 stars5/5 (2)
- Procedure - Context of The OrganizationDocument2 pagesProcedure - Context of The OrganizationBryant Castillo Gonzalez75% (4)
- 01 4.2.3 4.4.5 Document Control ProcedureDocument8 pages01 4.2.3 4.4.5 Document Control ProcedureYousaf RichuNo ratings yet
- 6.2document Control ProcedureDocument7 pages6.2document Control ProcedureStephen David Gozun100% (3)
- Context of The OrganizationDocument1 pageContext of The OrganizationBAla100% (4)
- 19-Procedure - Control of DocumentsDocument3 pages19-Procedure - Control of DocumentsAkshara Swamy100% (1)
- ISO 9001-2015 Implementation Plan Spreadsheet PDFDocument3 pagesISO 9001-2015 Implementation Plan Spreadsheet PDFAli ElbNo ratings yet
- Procedure - Context of The OrganizationDocument2 pagesProcedure - Context of The Organizationjaxf001No ratings yet
- Procedure - Control of DocumentsDocument5 pagesProcedure - Control of Documentsjamal nasirNo ratings yet
- 2.1 Control of Documented InfoDocument11 pages2.1 Control of Documented Infochaouch.najeh100% (2)
- 00 Procedure For Document and Record Control Preview enDocument3 pages00 Procedure For Document and Record Control Preview enashrafNo ratings yet
- Non Conformance ProceduresDocument2 pagesNon Conformance ProceduresHaroonAbdulRahim0% (1)
- Quality Record Procedure Rev-JDocument4 pagesQuality Record Procedure Rev-JherminNo ratings yet
- Control of Non-ConformanceDocument5 pagesControl of Non-ConformanceLawzy Elsadig SeddigNo ratings yet
- Control of Documents ProcedureDocument5 pagesControl of Documents Procedureaileen_macayanNo ratings yet
- Quality Procedure - Context of The OrganizationDocument5 pagesQuality Procedure - Context of The OrganizationAvyan Kelan100% (2)
- Procedure 01 - Control of Documents and RecordsDocument6 pagesProcedure 01 - Control of Documents and Recordssuhara hussainNo ratings yet
- Procedure Risk ManagementDocument2 pagesProcedure Risk ManagementAnbuNo ratings yet
- E Internal Audit Procedure Section 5Document3 pagesE Internal Audit Procedure Section 5Ngonidzashe Zvarevashe100% (1)
- ISO 9001, 14001 and 4001 Context of The OrganizationDocument8 pagesISO 9001, 14001 and 4001 Context of The OrganizationJordin SladekNo ratings yet
- Control of RecordsDocument3 pagesControl of Recordschahi100% (1)
- DSPI-EQP-01 Procedure For Control of Documented InformationDocument17 pagesDSPI-EQP-01 Procedure For Control of Documented InformationISODCC DSPI100% (4)
- Audit Check List (ISO 9001)Document12 pagesAudit Check List (ISO 9001)morshed_mahamud7055No ratings yet
- Context & Interested Party Analysis RIODocument7 pagesContext & Interested Party Analysis RIOMuhamad HaykalNo ratings yet
- QP02 Control of RecordsDocument4 pagesQP02 Control of RecordsDida Wellby100% (2)
- PR-5 - Docuent Control ProcedureDocument7 pagesPR-5 - Docuent Control ProcedureSAMEER JAVEDNo ratings yet
- Document Control ProcedureDocument6 pagesDocument Control ProcedureLedo Houssien93% (15)
- 04 Procedure For Internal QMS AuditDocument3 pages04 Procedure For Internal QMS AuditQualtic Certifications100% (4)
- F Control of Non - Conforming Product ProcedureDocument3 pagesF Control of Non - Conforming Product ProcedureNgonidzashe Zvarevashe100% (1)
- Document Control ProcedureDocument12 pagesDocument Control ProcedureBãoNo ratings yet
- Iso 9001 Toolkit File ListDocument3 pagesIso 9001 Toolkit File ListEl KhanNo ratings yet
- Control Document ProcedureDocument10 pagesControl Document ProcedurenizardsouissiNo ratings yet
- MRM AgendaDocument2 pagesMRM AgendaBAlaNo ratings yet
- Supplier Audit Check SheetDocument33 pagesSupplier Audit Check SheetMotive PostNo ratings yet
- QMS Nonconformity and Corrective Action ProceduresDocument11 pagesQMS Nonconformity and Corrective Action ProceduresNixNo ratings yet
- Management Review ProcedureDocument1 pageManagement Review ProcedureBAla83% (6)
- IMSP 01 Control of DocumentsDocument8 pagesIMSP 01 Control of Documentsemeka20120% (1)
- Document Control Procedure ExampleDocument3 pagesDocument Control Procedure ExampleErich Kadow33% (3)
- Internal Audit ReportsDocument1 pageInternal Audit ReportsCQMS 5S DivisionNo ratings yet
- ISO 9001:2015 Procedure For Control of Documented InformationDocument9 pagesISO 9001:2015 Procedure For Control of Documented InformationQualtic Certifications100% (6)
- Sample - 2 Procedure For Management ReviewDocument6 pagesSample - 2 Procedure For Management ReviewKauser Kazmi100% (1)
- Control Sample ISO 9000Document9 pagesControl Sample ISO 9000spongemouse80% (5)
- Procedure - Context of The OrganizationDocument5 pagesProcedure - Context of The OrganizationMarjorie Dulay DumolNo ratings yet
- Iso 9001:2000 Sample Audit Plan Schedule (Registrar)Document0 pagesIso 9001:2000 Sample Audit Plan Schedule (Registrar)Muhammad ShafiNo ratings yet
- QMS ManualDocument40 pagesQMS Manualafifa kausar100% (1)
- Procedure - Control of RecordsDocument4 pagesProcedure - Control of RecordsMarjorie Dulay DumolNo ratings yet
- F4E-QA-102 Supplier Audit Implementation 296E7T v2 3Document18 pagesF4E-QA-102 Supplier Audit Implementation 296E7T v2 3Jai BhandariNo ratings yet
- Non Conformities Corrective ActionsDocument37 pagesNon Conformities Corrective ActionsLanilyn AngNo ratings yet
- QSP 09 - Recruitment & TrainingDocument5 pagesQSP 09 - Recruitment & TrainingVivek V100% (1)
- QSP 06 - Preventive ActionDocument5 pagesQSP 06 - Preventive ActionVivek VNo ratings yet
- QSP 05 - Corrective ActionDocument4 pagesQSP 05 - Corrective ActionVivek V100% (2)
- QSP 02 - Record Control ProcedureDocument5 pagesQSP 02 - Record Control ProcedureVivek V100% (1)
- SP 46Document118 pagesSP 46Nilabh RoyNo ratings yet
- Simona Pipes, FittingsDocument56 pagesSimona Pipes, FittingsKristy DavisNo ratings yet
- O&g 1001Document47 pagesO&g 1001sultanthakurNo ratings yet
- Basic Heat TreatmentDocument12 pagesBasic Heat Treatmentpramod_goswamiNo ratings yet
- Lva1 App6892Document4 pagesLva1 App6892Vivek VNo ratings yet
- RWB 60-856 Inst - Op. Mantto.Document52 pagesRWB 60-856 Inst - Op. Mantto.Daniel Dennis Escobar Subirana100% (1)
- 10 AI Summer Vacation HWDocument2 pages10 AI Summer Vacation HWAyushi SinghNo ratings yet
- Vichinsky Et Al.2019Document11 pagesVichinsky Et Al.2019Kuliah Semester 4No ratings yet
- Haggling As A Socio-Pragmatic Strategy (A Case Study of Idumota Market)Document15 pagesHaggling As A Socio-Pragmatic Strategy (A Case Study of Idumota Market)Oshoja Tolulope OlalekanNo ratings yet
- What's New: Contemporary Quiz#5Document2 pagesWhat's New: Contemporary Quiz#5Christian Castañeda100% (1)
- Existing VendorsDocument1 pageExisting VendorsSuperintending EngineerNo ratings yet
- Very High Frequency Omni-Directional Range: Alejandro Patt CarrionDocument21 pagesVery High Frequency Omni-Directional Range: Alejandro Patt CarrionAlejandro PattNo ratings yet
- CasDocument2 pagesCasJamesalbert KingNo ratings yet
- Instructional Module: IM No.: IM-NSTP 1-1STSEM-2021-2022Document6 pagesInstructional Module: IM No.: IM-NSTP 1-1STSEM-2021-2022Princess DumlaoNo ratings yet
- Sec ListDocument288 pagesSec ListTeeranun NakyaiNo ratings yet
- Mind Surge NewDocument65 pagesMind Surge NewmazzagraNo ratings yet
- Sika Decap PDFDocument2 pagesSika Decap PDFthe pilotNo ratings yet
- Tankless Vs TankDocument2 pagesTankless Vs TankClick's PlumbingNo ratings yet
- B4 Ethical Problems and Rules of Internet NetiquetteDocument11 pagesB4 Ethical Problems and Rules of Internet NetiquetteTarkan SararNo ratings yet
- C PM 71.v2016-12-11 PDFDocument31 pagesC PM 71.v2016-12-11 PDFbobi2201No ratings yet
- Materi 2 - Obligation, Prohibition, and Suggestion - AdviceDocument12 pagesMateri 2 - Obligation, Prohibition, and Suggestion - AdviceShadrina ChaerunissaNo ratings yet
- S&S PDFDocument224 pagesS&S PDFMohammed MateenNo ratings yet
- 3838-Article Text-7786-1-10-20230403Document8 pages3838-Article Text-7786-1-10-20230403Ramona Elena SpiridonNo ratings yet
- Comparisons YouTrackDocument13 pagesComparisons YouTrackMihai DanielNo ratings yet
- All I Need Is Love by Klaus Kinski 0394549163 PDFDocument5 pagesAll I Need Is Love by Klaus Kinski 0394549163 PDFFernanda ArzaguetNo ratings yet
- Resume FixedDocument2 pagesResume Fixedapi-356691606No ratings yet
- SAP2000 Analysis - Computers and Structures, IncDocument6 pagesSAP2000 Analysis - Computers and Structures, IncshadabghazaliNo ratings yet
- Collimation of Binoculars With A LampDocument33 pagesCollimation of Binoculars With A LampchristianNo ratings yet
- Assessment of Spinach Seedling Health Status and Chlorophyll Content by Multivariate Data Analysis and Multiple Linear Regression of Leaf Image FeaturesDocument9 pagesAssessment of Spinach Seedling Health Status and Chlorophyll Content by Multivariate Data Analysis and Multiple Linear Regression of Leaf Image FeaturesYugal KumarNo ratings yet
- 6 The Relationship Between Job Satisfaction and Intention To Stay in Taiwanese Nurse PractitionersDocument1 page6 The Relationship Between Job Satisfaction and Intention To Stay in Taiwanese Nurse Practitionersroselle portudoNo ratings yet
- Validation of Analytical ProceduresDocument15 pagesValidation of Analytical ProceduresildamonalisaNo ratings yet
- Winning at New ProductsDocument24 pagesWinning at New Products劉緯文100% (1)
- Florian1995, Mental HealthDocument9 pagesFlorian1995, Mental Healthade ubaidahNo ratings yet
- The Law of CosinesDocument4 pagesThe Law of Cosinesapi-213604106No ratings yet
- CreditCardStatement3665658 - 2087 - 02-Mar-21Document2 pagesCreditCardStatement3665658 - 2087 - 02-Mar-21Aamir MushtaqNo ratings yet