Professional Documents
Culture Documents
R. K. Malik'S Newton Classes Ranchi
Uploaded by
Vikas NagarOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
R. K. Malik'S Newton Classes Ranchi
Uploaded by
Vikas NagarCopyright:
Available Formats
R. K. MALIK’S JEE (MAIN & ADV.
), MEDICAL
R. K. MALIK'S NEWTON CLASSES, RANCHI NEWTON CLASSES + BOARD, NDA, FOUNDATION
R. K. MALIK'S NEWTON CLASSES, RANCHI
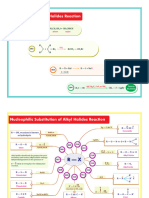
Summary Sheet #7 - Identifying the Patterns in Carbonyl Reaction Mechanisms
O Reactions of neutral NR2 NR2
HO R nucleophiles with
Grignard Reaction 12A P R X SN2 D
R–MgX alkyl halides R R R
R R R R
Reactions of HO RO
O anionic nucleophiles R X R OR R OH R CN SN2
HO CN
Cyanohydrin NaCN 12A P with alkyl halides CN
formation R R R R
O base O
O Enolate alkylation R X
Ketone or aldehyde HO H D SN2
NaBH4 (ketones or esters) R CH2R R R
reduction 12A P
or LiAlH4 R R R R R
O O O OH O
base HO H
Base-catalyzed aldol 12A 12E 12A P
D 12A P Reduction of esters LiAlH4
reaction R CH2R H R R R R OR R H
R
Addition of neutral O O
nucleophiles to acid RNH2 Grignard Reaction O HO R
12A 12E D + acid chloride, RMgX 12A 12E 12A P
halides or anhydrides R Cl R NHR
(e.g.amines, alcohols, water) ester, or anhydride R Cl R R
HI S S
O O O O R
O acid N
Addition of anionic nucleophiles HO RO
12A 12E Imine formation RNH2 P 12A PT 12E D
to acid halides or anhydrides
ES
R Cl H2N R OH R OR R NH2 R R R R
(e.g.RO , H2N , HO )
NC A K'
O O O O O acid O
base PT D
Claisen condensation D 12A 12E Fischer esterification ROH P 12A 12E
R CH2R R OR R R R OH R OR
R
O O
O R2CuLi O acid
1,4 addition of Gilman Amide hydrolysis H2O P 12A PT 12E D
14A P R NR2 R OH
reagent R R
RA C I R
O O O O O acid RO OR
base
Formation of acetals P 12A PT 12E 12A D
S
Michael reaction D 14A P ROH
R CH2R R R R R R R R
AL
R
O O RO OR acid O
Hydrolysis of acetals H2O P 12E 12A PT 12E D
RNH2 14A PT R R R R
1,4 addition of R R
neutral nucleophiles
RHN
O O acid O
L
Acid catalyzed aldol P T 12A PT T 14E D
R CH2R H R R R
ON M
The Nine Mechanistic Components (with examples)
[1,2] addition [1,2] elimination SN2 Deprotonation Proton Transfer
O O O O O
O Nu O base Intramolecular acid-base reaction
R H
WT .
12A O Nu 12E SN2 R D PT
R H3C R H2C R example:
X R X R X R Nu R
Cα X H
Nu Removal of a proton from substrate H
HO OR H2O OR
K
[1,4] addition Protonation R R R R
[1,4] elimination Keto-Enol Tautomerization
O O H O acid O
OH O O OH
14A Nu P
14E T H2C R H3C R
R R H
R R H3C R R
Nu H Addition of a proton to substrate
Nu X β
.
MAKE YOUR CONCEPTS CRYSTAL CLEAR
VISIT OUR YOU TUBE CHANNEL : MATHEMATICS MADE INTERESTING
Office.: 606 , 6th Floor, Hariom Tower, Circular Road, Ranchi-1,
Ph.: 0651-2562523, 9835508812, 8507613968
th
Office.: 606 , 6 Floor, Hariom Tower, Circular Road, Ranchi-1, Ph.: 0651-2562523, 9835508812, 8228920542
You might also like
- Identifying The Patterns in Carbonyl Reaction Mechanisms: R R NR NRDocument1 pageIdentifying The Patterns in Carbonyl Reaction Mechanisms: R R NR NRdjdjdhNo ratings yet
- Identifying The Patterns in Carbonyl Reaction Mechanisms: R R NR NRDocument1 pageIdentifying The Patterns in Carbonyl Reaction Mechanisms: R R NR NRoscar riosNo ratings yet
- REDUCTIONS FinalDocument11 pagesREDUCTIONS Finalgamer boomerNo ratings yet
- Essentials 2Document2 pagesEssentials 2aNo ratings yet
- Synthesis of Metal Complexes of Hydroxamic AcidDocument1 pageSynthesis of Metal Complexes of Hydroxamic AcidChannal Saif100% (1)
- Heterocycles Essentials2-2009Document2 pagesHeterocycles Essentials2-2009Aravindan NatarajanNo ratings yet
- Functional Group Reactions: C Synthesis Strategies, Chem 315/316 / Beauchamp 1Document19 pagesFunctional Group Reactions: C Synthesis Strategies, Chem 315/316 / Beauchamp 1Zia urRehman100% (1)
- Hetero Cyclic Handout 2Document2 pagesHetero Cyclic Handout 2anil_panmandNo ratings yet
- FunctionalGroups AnswersDocument2 pagesFunctionalGroups AnswerstaylorNo ratings yet
- 2016 Delt Paper Revision Accepted SupplementaryDocument11 pages2016 Delt Paper Revision Accepted SupplementarypufuNo ratings yet
- Grupos Funcionais Organicos (Ingles)Document1 pageGrupos Funcionais Organicos (Ingles)Jefferson RibeiroNo ratings yet
- Pauson Khand ReaktionDocument2 pagesPauson Khand ReaktionOrigamist KryaNo ratings yet
- 2 OxidationDocument28 pages2 Oxidationaggelisgeorge8546No ratings yet
- Antisense Oligonucleotide Biotechnology, Applications and FutureDocument29 pagesAntisense Oligonucleotide Biotechnology, Applications and FuturesurojitarpitaNo ratings yet
- ? Organic ChemistryDocument1 page? Organic ChemistryVineet KumarNo ratings yet
- GABA Barbiturates2002Document15 pagesGABA Barbiturates2002biqilaadengNo ratings yet
- C-C Bond Formation: M.C. White, Chem 253 Cross Coupling - 84-Week of October4, 2004Document36 pagesC-C Bond Formation: M.C. White, Chem 253 Cross Coupling - 84-Week of October4, 2004Mohammed AltahirNo ratings yet
- Synthesis and Evaluation of Antioxidant Activity of Semicarbazone DerivativesDocument5 pagesSynthesis and Evaluation of Antioxidant Activity of Semicarbazone DerivativesWalid EbaiedNo ratings yet
- Sach Giai Bai Tap 1 - 11Document22 pagesSach Giai Bai Tap 1 - 11Son Nguyen ThiNo ratings yet
- Heterocycle 16 PDFDocument7 pagesHeterocycle 16 PDFJesus NolascoNo ratings yet
- IOC Class-9 NotesDocument22 pagesIOC Class-9 Notesmardarchod 123No ratings yet
- REaction MechanismsDocument5 pagesREaction MechanismstaizokaiNo ratings yet
- Nucleophilic Substitution (S 1/S 2) Elimination (E1/E2)Document5 pagesNucleophilic Substitution (S 1/S 2) Elimination (E1/E2)Marck LyonNo ratings yet
- Enantioselective (3+3) Atroposelective Annulation Catalyzed by N-Heterocyclic CarbenesDocument10 pagesEnantioselective (3+3) Atroposelective Annulation Catalyzed by N-Heterocyclic CarbenesKatrin MarchenkoNo ratings yet
- Kap 12,13,17, IIDocument12 pagesKap 12,13,17, IImuraliNo ratings yet
- Organic Synthesis. Functional Group InterconversionDocument57 pagesOrganic Synthesis. Functional Group InterconversionJennifer Carolina Rosales NoriegaNo ratings yet
- Mechanism of DBTO CatalystDocument8 pagesMechanism of DBTO Catalystsahajahan shaikhNo ratings yet
- C C Bond Formation: From Other AcetylenesDocument4 pagesC C Bond Formation: From Other AcetylenesrashidNo ratings yet
- Important Reactions of Alkyl HalidesDocument3 pagesImportant Reactions of Alkyl HalidessusankmadiNo ratings yet
- Antonio P. Hache // Public School Flagship Store // Philadelphia, Pa // Design Studio BDocument6 pagesAntonio P. Hache // Public School Flagship Store // Philadelphia, Pa // Design Studio Bapi-26011247No ratings yet
- Approximate NMR Shift RangesDocument1 pageApproximate NMR Shift RangesashmaroofNo ratings yet
- Hydrocarbon: GMP GRDocument30 pagesHydrocarbon: GMP GRVinod AgrawalNo ratings yet
- Advanced Organic Reactions 2000 - WarrenDocument174 pagesAdvanced Organic Reactions 2000 - Warrenshiv57100% (3)
- SelectivityDocument4 pagesSelectivitySamik BiswasNo ratings yet
- Advances in Asymmetric Organocatalysis Over The Last 10 YearsDocument5 pagesAdvances in Asymmetric Organocatalysis Over The Last 10 YearsquimicosorioNo ratings yet
- Benzodiazepines andDocument49 pagesBenzodiazepines andryrahulyadav4No ratings yet
- Reactions of 3 - (4-Aryl-2-Thiazolyl) - and 3 - (2-Benzothiazolyl) - 2-Iminocoumarins With N-NucleophilesDocument8 pagesReactions of 3 - (4-Aryl-2-Thiazolyl) - and 3 - (2-Benzothiazolyl) - 2-Iminocoumarins With N-Nucleophileslabsoa111No ratings yet
- Diels Alder Reaction Strategy To Synthesize 1,2,3,6-Tetrahydro-1,2,4,5-Tetrazines Anti-Inflammatory Azoderiv !Document8 pagesDiels Alder Reaction Strategy To Synthesize 1,2,3,6-Tetrahydro-1,2,4,5-Tetrazines Anti-Inflammatory Azoderiv !Nickly NickNo ratings yet
- Bohlmann-Rahtz Pyridine Synthesis PDFDocument1 pageBohlmann-Rahtz Pyridine Synthesis PDFAbdullah Sabry AzzamNo ratings yet
- Essential Organic Chemistry Paula Yurkanis Bruice Full ChapterDocument67 pagesEssential Organic Chemistry Paula Yurkanis Bruice Full Chaptermargaret.jones429100% (6)
- Synthesis of Commercial Drugs 2011-12 - M2 HanoiDocument28 pagesSynthesis of Commercial Drugs 2011-12 - M2 HanoiCy MoonNo ratings yet
- Iit Reductions PDFDocument71 pagesIit Reductions PDFAshish SinghNo ratings yet
- Alcohols: Mind MapsDocument1 pageAlcohols: Mind Mapsbnnwdqwjz5No ratings yet
- Chem-353-Lecture 2Document10 pagesChem-353-Lecture 2Caleb AsharleyNo ratings yet
- Heterocycles Essentials3-2009Document2 pagesHeterocycles Essentials3-2009Aravindan NatarajanNo ratings yet
- Pyrene 2Document5 pagesPyrene 2Arjun paudelNo ratings yet
- Analysis of US FDA Approved Drugs Containing Sulfur Atoms: Kevin A. Scott Jon T. NjardarsonDocument34 pagesAnalysis of US FDA Approved Drugs Containing Sulfur Atoms: Kevin A. Scott Jon T. NjardarsonRead WhiteNo ratings yet
- Substitution V EliminationDocument25 pagesSubstitution V Eliminationsourish kumarNo ratings yet
- Instrumentación Electrónica: S&K S&K MFBDocument2 pagesInstrumentación Electrónica: S&K S&K MFBcbetancourth_35No ratings yet
- A. C-C Bond Forming Reactions: 04/05/2017 - Lecture:11-12Document39 pagesA. C-C Bond Forming Reactions: 04/05/2017 - Lecture:11-12asif MehmoodNo ratings yet
- A Memorandum for the President of the Royal Audiencia and Chancery Court of the City and Kingdom of GranadaFrom EverandA Memorandum for the President of the Royal Audiencia and Chancery Court of the City and Kingdom of GranadaNo ratings yet
- CAT Paper 2016..Document32 pagesCAT Paper 2016..Vikas NagarNo ratings yet
- S Block Notes PDFDocument29 pagesS Block Notes PDFVikas NagarNo ratings yet
- Test 11th 12th Sept 17 (ADVANCE Chem)Document8 pagesTest 11th 12th Sept 17 (ADVANCE Chem)Vikas Nagar100% (1)
- S Block Notes (IIT-JEE & NEET)Document29 pagesS Block Notes (IIT-JEE & NEET)Vikas NagarNo ratings yet
- Surface Chemistry Resonance NoteDocument8 pagesSurface Chemistry Resonance NoteSomya Kumar SinghNo ratings yet
- HydrogenDocument12 pagesHydrogenVikas NagarNo ratings yet
- Test 11th 12th Sept 17 (MAINS Chem)Document3 pagesTest 11th 12th Sept 17 (MAINS Chem)Vikas NagarNo ratings yet
- Viii Nstse Mock Test # 02Document21 pagesViii Nstse Mock Test # 02Vikas NagarNo ratings yet
- Revision Notes On Chemical BondingDocument11 pagesRevision Notes On Chemical BondingVikas NagarNo ratings yet
- Casting DefectsDocument61 pagesCasting DefectsVikas NagarNo ratings yet
- Flame SynthesisDocument48 pagesFlame SynthesisVikas NagarNo ratings yet
- Flame SynthesisDocument48 pagesFlame SynthesisVikas NagarNo ratings yet
- Industrial Training Report (Training at Yamaha Motors) by Vikas NagarDocument42 pagesIndustrial Training Report (Training at Yamaha Motors) by Vikas NagarVikas Nagar75% (8)
- Virtual Baja Design ReportDocument1 pageVirtual Baja Design ReportVikas NagarNo ratings yet
- Designing A BAJA SAE Vehicle 3-10-2007!7!12 24 PMDocument10 pagesDesigning A BAJA SAE Vehicle 3-10-2007!7!12 24 PMMainakNo ratings yet
- O o o o o o o o o O: Sf-Announce Sf-DiscussDocument21 pagesO o o o o o o o o O: Sf-Announce Sf-DiscussVikas NagarNo ratings yet