Professional Documents
Culture Documents
Solid Solutions and Phase Diagrams Explained
Uploaded by
Saniya SohailOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Solid Solutions and Phase Diagrams Explained
Uploaded by
Saniya SohailCopyright:
Available Formats
Solid Solutions/Phase Diagrams C.
C. Nitriding: T < A1, Gas nitriding: only N, Carbonitriding: C and N both Modifiers: reduce melting temperature and viscosity for easy processing
Alloy: metal made with a pure metal + other elements diffuse to surface, Cyaniding – immerse in liquid cyanide (both C and N)
Components: elements that make an alloy Weldability of Steel: (a) Structure at Tmax (b) After cooling in a steel of low Glass Heat Treatment:
Phase: Same structure, atomic arrangement, same composition, hardenability (c) After cooling in a steel of high hardenability. Metal - Stress-relief Annealing: Heat up to annealing point and cool down
properties. A definite interface bw phase and surrounding phases. Zero Low C steels 1) Weld easily, 2) the strength of welded mater is greater slowly to reduce or eliminate the residual stress
DOF means you cant change the pressure or temp on a phase diagram than matrix (good). 3) Ductile, 4) Hard precipitates 5)Low hardenability Annealed Glass (no or little stress)
and vice versa. Medium-high C steels Form martensite easily after cooling of HAZ – brittle Tempered Glass (Compressive stress
Solid Solution: One or more solutes in solvent(solid). Not a compound, failure Glass Ceramics: Crystalline materials derived from amorphous glasses.,
crystal structure unchanged, single homogeneous phase. In Unlimited Stainless steels contain a minimum of 11% C, which permits a thing, Good mechanical strength/toughness, corrosion resistance at high
solubility, only one phase is produced, NOT A MIXTURE. Size is an imp protective surface layer of chromium oxide. Cr stabilizes ferrite phase; Ni temperature
factor! Hume Rothery Rule: size <15% diff in diameter, crystal structure stabilizes austenite phase. Types: Clay Products: Clays are hydrous sheet aluminum silicates with a
should be same, valence same, electronegativity same. In Limited Ferritic stainless steels :< 30% Cr, < 0.12% C, BCC, Good strength, crystalline structure, Use water as a binder for ceramic particles (silica),
solubility excessive atoms form a compound, two solid phases exist. FCC austenite (<0.2%C) BCC martensite moderate ductility, Ferromagnetic, not heat treatable Common applications: Pottery/bricks Forming techniques: clay -> liquid
Excess material forms cluster. FCC austenite (higher C) Body-centered-tetragonal martensite (no Martensitic stainless steels: < 17% Cr, 0.1-1% C, High hardness, corrosion phase formed -> glassy bond.
Solid Soln. Hardening: Increase in strength, hardness, creep resistance closed-packed plane, very hard) resistant, Applications: knives, ball bearings, valves Refractories: High melting-point ceramics, Used in production refining,
(high temp alloy are SS). Decrease in electrical conductivity. Plate martensite is harder due to more dislocations and crystal Austenitic stainless steels: Excellent ductility, formability, corrosion handling of metals/glasses., Three types: Acidic, Basic, and Neutral
Binary Isomorphous System: Liq phase is homogeneous, alpha phase is structure. resistance, Non-magnetic (good for cardiovascular stent), Can be cold Ceramic Alloys: Metal alloys: strengthening, corrosion resistance,
solid soln. If pressure is constant, can change the temp and composition. Tempering of Martensite: Martensite is a non-equilibrium phase, not worked to achieve higher strength, 304 stainless steel (18Cr-8Ni) – Ceramic alloys: sintering to full density, fracture toughness.
Lever Rule: % x = (%A in alloy)-(%A in Liquid) / (%A in alpha) –(%A in shown on the phase diagram. Tempering releases the carbon atoms to medical grade High-Performance Ceramics:
Liquid)….. Use Tie line to do this. form α + cm. Becomes less hard but more ductility (toughness). Precipitation hardened (PH) stainless steel, e.g.,17Cr-4Ni (17-4 PH), Diamond: the hardest material. Industrial applications include coating,
Micro segregation: B/w dendrites, can be fixed by homogenization Contain Al, Nb Ta, Processing: A, quench to form M, temp to form Ni3Al abrasives (grinding/polishing)
Homogenization: Heat up below non-equil. solidus to promote diffusion Heat Treatment of Steels Duplex stainless steels: 50% Austenite + 50% Ferrite SiC: good oxidation resistance at high temperatures. Applications include
Macro segregation: b/w surface and center, fixed by hot working. 3 important microstructures = pearlite, bainite, tempered martensite. Cast Irons: Eutectic undercooled by 6C, graphite becomes Fe3C white coating, abrasives(grinding wheels),
Heat Treatments of HYPOEUTECTOID Steels: iron, Bi – bismuth, Silicone reduces the amount of carbon contained in the
Dispersion Strengthening & Eutectic Phase Diagrams: -Process annealing (80-170oC below A1): remove residual stresses eutectic. Carbone equivalent (CE) = %C + 1/3%Si, 4.3%CE forms eutectic. Intermetallic Compound: contains two or more metallic elements,
-Austenitizing: Heat to above A3 to produce full austenite Annealing soft ferritic matrix (not coarse pearlite as in steels!) producing a new phase with its own composition, crystal structure, and
-Annealing (Austenitizing at 30oC above A3, slow cool): coarse pearlite Austempere bainite + graphite properties. Intermetallic compounds are almost always very hard and
-Normalizing (Austenitizing at 55oC above A3, rapid cool in air): fine Normalizing pearlite + graphite brittle.
pearlite Gray Cast Iron: Small interconnected graphite flakes (eutectic cells), Low Stoichiometric intermetallic compounds have a fixed composition. On
Heat Treatments For HYPEREUTECTOID Steels: strength, low ductility, Good thermal fatigue resistance, vibration the phase diagram they have a vertical line.
-Austenitizing: Heat to above A1 to γ and Fe3C damping, thermal conductivity Nonstoichiometric intermetallic compounds have a range of
-Annealing (at 30oC above A1, slow cool): coarse pearlite White Cast Iron: Contains lots of Fe3C (instead of graphite), Hard, Brittle, compositions and are sometimes called intermediate solid solutions.
-Normalizing (55oC above Acm, rapid cool): fine pearlite Highly alloyed white irons have good resistance to abrasive wear, due to Solid Reactions: Solid to Solid
-Spheroidizing (30oC below A1, long time annealing): spherical Fe3C, alloy carbides and martensite. First solid to form, draw a tie line from liquid to solid region.
ductile (improved machinability) Malleable Cast Iron: Malleable iron is formed by the heat treatment of Final Microstructure cooled from liquid contains pieces of gamma and
Isothermal Heat Treatments white cast iron……Clumps of graphite, Better ductility than gray and lamellar structure around it.
-Isothermal annealing: above nose temp, produces pearlite while cast irons, very machinable, Ferritic malleable iron –Slow cooled
-Austempering: below nose temp, produces bainite Pearlitic malleable iron – Fast cooled in air or oil Artificial Age Hardening for Cu 10% Be alloy. 1)Solution treat bw 605-
Hypoeutectic: alloy with a composition bw the left end of the tie line Quenching and Tempering: Retained Austenite Ductile (Nodular) Cast Iron: CE ~ 4.3% with Mg., Contains spheroidal 850C. 2) Quench. 3)Age below 605(around 400).
defining the eutectic reaction and composition. Area left of 0.8. -Quenching: hardening graphite particles, Better strength and ductility compared to gray iron and
Hypereutectic: alloy composition bw the right-hand end and defining -Tempering: regain toughness, ductility malleable irons., Malleable iron has better fracture toughness than Cold Work= A_initial – A_final / A_initial
the eutectic composition & reaction. Area right of 0.8. -Retained austenite Nodular iron.
Strength of Eutectic Allows: Finer colony is better, interlamellar spacing -How: Austenite does not fully transform to martensite. Compacted Graphite Iron: Contains rounded but interconnected Cr is important in steels because H is a ferrite stabilizes and impacts
leads to faster solidification = finer = stronger, depends on amount of -Problem: After tempering of martensite, during cooling, retained graphite, Advantages of both Gray and Ductile irons: Strengths, ductility, stainless character to the steel
eutectic. austenite transforms to brittle martensite thermal conductivity, damping Mn is imp because H alters the phase diagram, reduces eutectoid temp
Primary Phase Refinement: Done by adding 0.05% Phosphorus to -Solution: need a second tempering and composition. It lowers the Cr requirement since it acts like a
encourage nucleation. Residual Stress: Induces Cracking. Surface cools faster, forms Ceramics: Ceramics may be crystalline, partially crystalline, or amorphous austenite stabilizer.
martensite. When austenite becomes martensite, expands and induces Ionic: typically metals and non-metals (e.g., NaCl, MgO, Al2O3)
Phase Transformations & Heat Treatment: tensile stress on surface, which leads to cracking. Covalent: metalloids and non-metals or pure elements, (SiO2, SiC,
Remove Surface Crack: Marquenching or Martempering, hold until the Diamond
temperature equalizes in the steel. Properties: Generally brittle but may behavior plastic under low strain
Purpose of alloying: solid solution strengthening, form alloy carbide rate and high temperature , Generally stiffer than metals, Density is often
Arrhenius relationship => Growth rate= A*exp(-Q/RT) precipitates (rather than brittle Fe3C); improve corrosion resistance; low (due to porosity), Most ceramics are intrinsically hard: Covalent bond
Lower T, Low T, higher nucleation, low diffusion fine grains improve hardenability (changes H.T. curve); reduce carbon content, produces resistance to dislocation, Ionic bond structures produce
High T, higher diffusion, low nucleation large grains phase stability electrostatic repulsion of opposite charged ions
Incoherent: Matrix is intact Hardenability: The ease to form martensite (in thick section of steels): a) Flaws: Porosity increases, strength decreases
Coherent: Matrix is distorted – more useful Plain carbon steel – low hardenability (difficult to form marten., need Apparent porosity =
Guinier-Preston (GP) zones: Tiny clusters of atoms that precipitate from very high cooling rate), b) Alloy steel – better hardenability
the matrix in the early stage of age hardening Ausforming – increase the strength without an adverse effect on
Good Age-Hardenability: Substantial solid solution region, Decreasing toughness.
solid solubility with decreasing temperature, Jominy test: Jominy test – compare hardenability of steels
Soft matrix and hard precipitates, Quenchable, Coherent precipitates Jominy distance – distance from the quenched end
Al alloys: Hardenable : copper, Mg+Si , Zn
Grossman Chart: Used to determine the hardness at the center of a
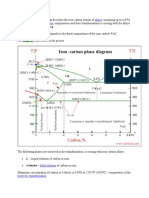
Fe-C System = Eutectoid Reaction round bar, Used to determine the need for different quenching methods
α-Fe: Ferrite, γ-Fe: Austenite, Iron Carbide: Cementite Surface Treatments:
0.8 is eutectoid point A. Case Hardening: Make surface strong – fatigue, wear resistance, Inorganic glass: Soda-lime glass, potash-lime glass, lead-glass (all contain
Controlling of eutectoid reaction: High strength is associated with: The Heating surface (T > A3), then cool down – Martensite on surface, soft Silica), Non-crystallization (amorphous), Metastable, hard/Brittle,
amount of carbon, Size of pearlite colonies, Size of the lamellar ferrite core, Heating technique: gas flame, induction coil, laser beam. Undercooled liquid – Density slope changes at Tg No transition at Tm
structure, Transformation temperature B. Carburizing: T > A3, C diffuse into surface – hardened surface, Much
thinner surface layer than heating induced surface layer Glass Former/Modifier: Formers: base material, amorphous
Intermediates: prevent thermal shock
You might also like
- Chapter 4 Solid Solution Equilibrium Phase Diagram PDFDocument41 pagesChapter 4 Solid Solution Equilibrium Phase Diagram PDFSergio Syamil100% (2)
- Iron Carbon Phase DiagramDocument3 pagesIron Carbon Phase DiagramrabikmNo ratings yet
- Metallic Materials Sessional Microstructure StudyDocument39 pagesMetallic Materials Sessional Microstructure StudyMuhammedNayeemNo ratings yet
- Engineering Alloys (Non Ferrous)Document52 pagesEngineering Alloys (Non Ferrous)Sukhwinder Singh GillNo ratings yet
- Iron Carbon Diagram of Steel PDFDocument6 pagesIron Carbon Diagram of Steel PDFshihabscb1971100% (1)
- 23 - Toughened Ceramics 1Document28 pages23 - Toughened Ceramics 1Md. Rafiqul IslamNo ratings yet
- Chapter 6 Phase DiagramsDocument73 pagesChapter 6 Phase DiagramsSaiful AzrieNo ratings yet
- 20230116-MT-205-PD-BNS-L-4 To L-6 (2022-2023) Notes - 2Document47 pages20230116-MT-205-PD-BNS-L-4 To L-6 (2022-2023) Notes - 2Kaustav SaikiaNo ratings yet
- Selective LeachingDocument13 pagesSelective LeachingMuhammad MohtashimNo ratings yet
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanNo ratings yet
- CorrosionNotes Handout1 2017 v1 PDFDocument40 pagesCorrosionNotes Handout1 2017 v1 PDFAbdo MohdyNo ratings yet
- 4 Unit - Heat Treatment of SteelsDocument72 pages4 Unit - Heat Treatment of SteelsAnway WalkeNo ratings yet
- Engineering Corrosion OH-4: University of Hafr Al BatinDocument41 pagesEngineering Corrosion OH-4: University of Hafr Al BatinHussain Al-DawoodNo ratings yet
- SolidificationDocument10 pagesSolidificationAnonymous RY3dAWN9o100% (1)
- The Iron-Carbon Phase Diagram: Prof. H. K. Khaira Professor in MSME Deptt. MANIT, BhopalDocument38 pagesThe Iron-Carbon Phase Diagram: Prof. H. K. Khaira Professor in MSME Deptt. MANIT, BhopalYogesh KumbharNo ratings yet
- Casting Technology 04Document11 pagesCasting Technology 04Sreekumar RajendrababuNo ratings yet
- Heating Element ArticleDocument10 pagesHeating Element ArticleFrea Kent-Dazze D'DrughiNo ratings yet
- Lecture 04-CCT and TemperingDocument17 pagesLecture 04-CCT and TemperingRudy Dwi PrasetyoNo ratings yet
- Heat TreatmentDocument21 pagesHeat TreatmentChernet MerknehNo ratings yet
- Assignment 2Document1 pageAssignment 2Laura RobayoNo ratings yet
- Corrosion Rate Calculation PDFDocument10 pagesCorrosion Rate Calculation PDFcometNo ratings yet
- W6 Lecture 6.surface Hardening of Steel PDFDocument28 pagesW6 Lecture 6.surface Hardening of Steel PDFYota KimireNo ratings yet
- Weld Metal Solidification-1 - Grain StructureDocument51 pagesWeld Metal Solidification-1 - Grain StructureChelekara Subramanian Abhilash Iyer100% (3)
- Failure Analysis of Cracked Reformer Tubes Reveals Thermal Shock CauseDocument6 pagesFailure Analysis of Cracked Reformer Tubes Reveals Thermal Shock CauseOwais MalikNo ratings yet
- Basic Metallurgy: Numbering Systems For Metals and AlloysDocument15 pagesBasic Metallurgy: Numbering Systems For Metals and AlloysAcid BurnsNo ratings yet
- Martensitic Stainless SteelsDocument8 pagesMartensitic Stainless SteelsAdilmar E. NatãnyNo ratings yet
- lectut-MTN-513-pdf-Structure of Crystalline CeramicsDocument53 pageslectut-MTN-513-pdf-Structure of Crystalline CeramicsAkash AgarwalNo ratings yet
- wk7 (3) - Fe-C SystemDocument12 pageswk7 (3) - Fe-C Systemsaeed khaledNo ratings yet
- Chap 6 TTT Diagram (New)Document26 pagesChap 6 TTT Diagram (New)eeit_nizamNo ratings yet
- Recovery Recrystallization Grain GrowthDocument15 pagesRecovery Recrystallization Grain Growthteju1996coolNo ratings yet
- Segregation and Banding in SteelDocument2 pagesSegregation and Banding in SteelskluxNo ratings yet
- Duplex SSDocument12 pagesDuplex SSTushar PatilNo ratings yet
- Heat Treatment Properties and ProcessesDocument70 pagesHeat Treatment Properties and ProcessesAbdulmhsen ALjreedan100% (1)
- Chapter 13 - Heat Treatment of SteelsDocument60 pagesChapter 13 - Heat Treatment of SteelsRecep VatanseverNo ratings yet
- 5 Iron-Cementite Phase DiagramDocument46 pages5 Iron-Cementite Phase DiagramsmrutiNo ratings yet
- Lecture2 Diffusion IN SOLIDSDocument72 pagesLecture2 Diffusion IN SOLIDSidownloadbooks3133No ratings yet
- CarburisingDocument4 pagesCarburisingSelva KumarNo ratings yet
- Capitolo 5 - Laminate - enDocument40 pagesCapitolo 5 - Laminate - enkelvynNo ratings yet
- Corrosion Measurement UNIT-5: CHE-545-172 DR Ime B.ObotDocument48 pagesCorrosion Measurement UNIT-5: CHE-545-172 DR Ime B.ObotArielNo ratings yet
- Shaw ProcessDocument2 pagesShaw ProcessSuresh KumarNo ratings yet
- Materials Science Lec 04 Phase & Iron-Carbon DiagramDocument53 pagesMaterials Science Lec 04 Phase & Iron-Carbon DiagramKrishna SarkarNo ratings yet
- Ferrite Limitation For SS316LDocument12 pagesFerrite Limitation For SS316LAntonio PerezNo ratings yet
- Two Step SinteringDocument4 pagesTwo Step Sinteringrajadasari5682No ratings yet
- ASTM Designation B221-12Document15 pagesASTM Designation B221-12Shahzad KhanNo ratings yet
- Fuels and Combustion: - Calorific Value - Significance and Comparison Between LCV andDocument46 pagesFuels and Combustion: - Calorific Value - Significance and Comparison Between LCV andSandhya SundarNo ratings yet
- 06-Fatigue and Creep of Materials - F17 PDFDocument23 pages06-Fatigue and Creep of Materials - F17 PDFsabavoonNo ratings yet
- Oil Field Alloy Selection GuideDocument11 pagesOil Field Alloy Selection GuideAnonymous 7yN43wjlNo ratings yet
- Powder MetallurgyDocument44 pagesPowder MetallurgyjrvinodNo ratings yet
- Case Study On Copper CorrosionDocument15 pagesCase Study On Copper CorrosionClaudia MmsNo ratings yet
- Aluminium Titanate: Chemical FormulaDocument6 pagesAluminium Titanate: Chemical FormulaaadhanNo ratings yet
- Nickel & Special HT steels for Petrochemical ApplicationsDocument39 pagesNickel & Special HT steels for Petrochemical ApplicationsganeshNo ratings yet
- Solidification, Phase Diagrams and Phase TransformationDocument35 pagesSolidification, Phase Diagrams and Phase TransformationkrishnasaiNo ratings yet
- Applying the lever rule to phase diagramsDocument4 pagesApplying the lever rule to phase diagramsMathaneshan RajagopalNo ratings yet
- Case Hardening: Surface Hardening TechniquesDocument14 pagesCase Hardening: Surface Hardening TechniquesmasoodNo ratings yet
- Precipitation-Hardening Stainless Steels: Properties and Types (Martensitic, Austenitic, SemiausteniticDocument3 pagesPrecipitation-Hardening Stainless Steels: Properties and Types (Martensitic, Austenitic, SemiausteniticClaudia MmsNo ratings yet
- Corrosion Resistance of High Nitrogen Steels PDFDocument27 pagesCorrosion Resistance of High Nitrogen Steels PDFAnil Kumar TNo ratings yet
- Transition Metal ToxicityFrom EverandTransition Metal ToxicityG. W. RichterNo ratings yet
- Kinetics Study of Ester Hydrolysis in The Presence of HCL at Room Temperature Room TemperatureDocument10 pagesKinetics Study of Ester Hydrolysis in The Presence of HCL at Room Temperature Room TemperatureJuan RobinsonNo ratings yet
- Introduction Palm Oil Processing - 2Document32 pagesIntroduction Palm Oil Processing - 2hantudonatNo ratings yet
- Science Rate of Reaction Vinegar + Baking SodaDocument3 pagesScience Rate of Reaction Vinegar + Baking SodaWalter Bastian Porcel Espinoza40% (5)
- Major ingredients and processing of margarineDocument3 pagesMajor ingredients and processing of margarinefazalNo ratings yet
- Smoke BombDocument8 pagesSmoke Bombkunjansingh431No ratings yet
- Thermal Insulation Barrier Providing Corrosion Protection With "Cool-To-Touch" PropertiesDocument2 pagesThermal Insulation Barrier Providing Corrosion Protection With "Cool-To-Touch" PropertiesnarmathaNo ratings yet
- EG0800300 UV-protection of Natural and Synthetic Fabrics by Surface Treatment Under The Effect of Gamma IrradiationDocument9 pagesEG0800300 UV-protection of Natural and Synthetic Fabrics by Surface Treatment Under The Effect of Gamma IrradiationariefNo ratings yet
- Standard Sheetmetal Slitting TolerancesDocument11 pagesStandard Sheetmetal Slitting TolerancessudarshanNo ratings yet
- 50 Oraciones de Ingles 1!Document3 pages50 Oraciones de Ingles 1!WJ-nimodo QuispeNo ratings yet
- BS en 14582-2016Document40 pagesBS en 14582-2016ASESORIAS SOLDADURAS100% (1)
- LED Flashlight Design for Circular EconomyDocument12 pagesLED Flashlight Design for Circular EconomyAlexander GhidellaNo ratings yet
- Parker O-Ring VM835-75 Compound Data SheetDocument2 pagesParker O-Ring VM835-75 Compound Data SheetBrandon TrocNo ratings yet
- H05667 HPGDocument4 pagesH05667 HPGHanesh MehtaNo ratings yet
- Numerical Chapter 1Document6 pagesNumerical Chapter 1RobinsNo ratings yet
- UntitledDocument6 pagesUntitledOmega Princess TayonaNo ratings yet
- HazMat IAP PlanDocument12 pagesHazMat IAP PlanVanitta RangsitananNo ratings yet
- 5-194 - Kru276 Eng PDFDocument1 page5-194 - Kru276 Eng PDFMoslemNo ratings yet
- J. Electrochem. Soc.-1952-Loonam-295C-8CDocument4 pagesJ. Electrochem. Soc.-1952-Loonam-295C-8CGeovanny JaenzNo ratings yet
- Principles of Major Manufacturing ProcessesDocument40 pagesPrinciples of Major Manufacturing ProcessesWatyu Dennis PeterNo ratings yet
- 4 Chapter Liquids and Solids McqsDocument6 pages4 Chapter Liquids and Solids McqsAáwáíź Jútt0% (1)
- DLVO Theory PDFDocument7 pagesDLVO Theory PDFshashank mishraNo ratings yet
- Chemistry Project: On "" Investigate Various Constituents of Coffee "Document12 pagesChemistry Project: On "" Investigate Various Constituents of Coffee "arnav100% (1)
- Typical Mechanical Properties (As Welded) : Do Not Breathe Fumes!Document20 pagesTypical Mechanical Properties (As Welded) : Do Not Breathe Fumes!Samuel LatumahinaNo ratings yet
- The Chemistry of COSDocument20 pagesThe Chemistry of COSsharkkingkingNo ratings yet
- Pompe À Eun Honda WMP20XDocument100 pagesPompe À Eun Honda WMP20XLaure GarnierNo ratings yet
- Aerogel drying process for producing transparent insulationDocument6 pagesAerogel drying process for producing transparent insulationcarlette11No ratings yet
- Saunf Ajwaini ProjectDocument15 pagesSaunf Ajwaini Projectsatyam raj0% (1)
- Algae BiodieselDocument6 pagesAlgae BiodieselRanjit MarimuthuNo ratings yet
- AISI 4140 Alloy Steel PropertiesDocument3 pagesAISI 4140 Alloy Steel PropertiesSatrio AmiruddinNo ratings yet
- Formulation and Characterization of Sustained Release Dosage Form of Moisture Sensitive DrugDocument10 pagesFormulation and Characterization of Sustained Release Dosage Form of Moisture Sensitive DrugDIKANo ratings yet