Professional Documents
Culture Documents
Separation of Substances
Uploaded by
sangops0 ratings0% found this document useful (0 votes)
106 views4 pageseducation
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documenteducation
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
106 views4 pagesSeparation of Substances
Uploaded by
sangopseducation
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 4
SEPARATION OF SUBSTANCESS
Answer the following questions:
1. What are pure substances?
A. A pure substance is made up of only one kind of particles. It has definite ,
well defined set of properties and has the same composition throughout.
Examples: elements and compounds.
2. What is a mixture?
A. A mixture is made up of two or more substances. They do not have definite
and well defined properties.
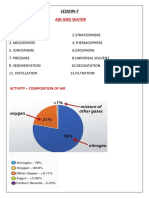
Examples: Air and water
3. List out the properties of mixtures.
A. Some important properties of mixtures are as follows:
(i) The composition of a mixture is not fixed.
(ii) Each component of the mixture retains its characteristic properties.
(iii) The substances present in a mixture can easily be separated.
(iv) Mixtures do not have a fixed melting and boiling points.
4. Define the following terms.
(a) Hand- picking: The process of removing unwanted components such as a
pebble, straw, etc from grains and pulses.
(b) Threshing: The process of beating out the harvested crops to separate
the grains from stalk. There are machines called threshers that can also be
used.
(c) Winnowing: The process of separating the grains from chaff or husk by
exposing to natural breeze.
(d) Sieving: The process of separating mixtures containing components of
different sizes, shapes and colours by using a sieve .
5. Name the apparatus that can be used to separate a mixture of mustard oil
and water. Explain its working.
A. (i) The apparatus used for separating mustard oil and water is Separating
funnel.
(ii) WORKING:
A) The mixture of oil and water is taken in a separating funnel and left
undisturbed for some time.
B) The heavier liquid (water) collects as the bottom layer and the lighter oil
forms the top layer.
C) A beaker is placed below the funnel to collect the liquid.
D) The stopper is opened to let the bottom liquid to flow out and turned off
just when the top layer of liquid reaches the mouth of the stopper.
Note : Draw the fig. 5.11 from text book pg. no. 57
6. Describe the process to separate a mixture of sand and water.
A. 1. The process involved in separating a mixture of sand and water are
sedimentation and decantation.
2. Make a mixture of sand and water in a beaker. Allow the mixtures to stand
for sometime undisturbed. The sand particles settle down at the bottom of the
beaker forming a sediment. The process of settling down of heavier, insoluble
particles from a mixture is called ‘ sedimentation’.
3. The clear liquid above the sediment is gently poured off into another
beaker without disturbing the sediment. This process of transferring the clear
liquid without disturbing the sediment is called ‘decantation’.
7. What is filtration ? How is it useful to us?
A. (i)Filtration is a process of removing insoluble substances from a liquid by
allowing it to pass through a filter paper. The clear fluid obtained is known as
Filtrate.
(ii) This method is used in our homes, laboratories and industries.
8. What is evaporation and condensation?
A. (i) The process in which a liquid changes into its vapour state is called
evaporation. This process is used on a large scale to obtain common salt from sea
water.
(ii) Condensation is a process of converting water vapour into water by cooling.
9. What do you understand by solution? Explain it in terms of solute and solvent.
A. (i) A solution is a homogenous mixture of one or more substances dissolved in
another substance.
(ii) The major component is called solvent and the minor component is called
solute.Example: In a solution of salt and water , salt is a solute and water is a
solvent.
10. Differentiate between saturated and unsaturated solutions.
A.
Saturated solutions Unsaturated solutions
Solutions which cannot dissolve more They are capable of dissolving more
solute at a given temperature solute at a given temperature.
11. How is drinking water obtained?
A. The water supplied to our houses is first treated through several separation
processes to make it clean and fit for consumption. The following steps are
followed to clean the water:
(i) The first step is removal of solid impurities by the process of sedimentation
and decantation. Sometimes, loading is done to speed up sedimentation.
(ii)The decanted water is then passed through sand filters to further purify it.
(iii) Finally, some amount of disinfectant ( chlorine) is added to kill the germs.
Note: Draw the fig. 5.12 from text book pg.no. 58.
12. Why do we need to separate mixtures?
A. Separation of mixtures into their components is done for the following
reasons:
(i) To remove impurities and harmful components.
(ii) To obtain useful components.
(iii)To obtain pure components.
13. Give reasons:
a) Loading is used to separate suspended impurities.
A) When alum is added to muddy water, the heavy particles of dissolved alum
settle on top of fine particles. This makes the fine particles heavy and they settle
down quickly. Thus, adding of alum loads the particles and speeds up
sedimentation.
b) Water is called a universal solvent.
A) (i)Water is capable of dissolving a variety of different substances, wherever it
goes, it takes valuable chemicals, minerals and nutrients dissolved it.
(ii) This property of water is very important to every living organism on earth and
therefore, it is called as the universal solvent.
14. State five functions of water.
A) Some of the crucial functions of water are as follows:
(i) Water helps in transporting vitamins and minerals in our body.
(ii) It is also used as a solvent to transport waste and unnecessary materials out of
the body, such as excess salt.
(iii) Plants take up vital nutrients, minerals and salts from soil through water.
(iv)Our body cannot absorb food if it is not dissolved in water.
(v) Aquatic plants and animals take up oxygen and carbondioxide
dissolved in water for sustaining life.
You might also like
- API 650-Water SS Tank-060914Document84 pagesAPI 650-Water SS Tank-060914Hamou MelloulNo ratings yet
- College of Education Long Quiz # 1: MixturesDocument7 pagesCollege of Education Long Quiz # 1: MixturesClaudia Inoc100% (1)
- Separating MixturesDocument8 pagesSeparating MixturesKes DimapilisNo ratings yet
- Chemical Equilibrium Multiple Choice QuestionsDocument4 pagesChemical Equilibrium Multiple Choice QuestionsCarol Mae Celis100% (5)
- Separation TechniqueDocument8 pagesSeparation TechniqueachoeyzNo ratings yet
- Introduction To Soil ScienceDocument18 pagesIntroduction To Soil ScienceMary Grace Nuñez SemillaNo ratings yet
- Https WWW - Elkem.com Global ESM Support Technical-Papers Refractories 37-The Use of Microsilica in Refractory CastablesDocument28 pagesHttps WWW - Elkem.com Global ESM Support Technical-Papers Refractories 37-The Use of Microsilica in Refractory CastablesSachin SahooNo ratings yet
- Class 6 CH 5 SolvedDocument8 pagesClass 6 CH 5 SolvedsayogitaNo ratings yet
- Chapter 5Document6 pagesChapter 5Abhinaba PaulNo ratings yet
- Separation of Substances: MixtureDocument5 pagesSeparation of Substances: MixtureShivam ZakhmiNo ratings yet
- Unit 3 Processes: Question and AnswerDocument5 pagesUnit 3 Processes: Question and AnswerAchmad NafisNo ratings yet
- Monté International School: Grade 7 Chemistry - Unit 5: WaterDocument7 pagesMonté International School: Grade 7 Chemistry - Unit 5: WaterjayapalNo ratings yet
- Lesson 2 Potable Water: To Explain How Water Is Made Safe To DrinkDocument18 pagesLesson 2 Potable Water: To Explain How Water Is Made Safe To Drinkjohn smithNo ratings yet
- E - Module of Grade-6. Separation of SubstancesDocument27 pagesE - Module of Grade-6. Separation of Substanceshree1202No ratings yet
- 2022 - 3NA - Separation Technique - NotesDocument19 pages2022 - 3NA - Separation Technique - NotesNitin Yadav Praduman (Qss)No ratings yet
- Water - A Wonderful LiquidDocument6 pagesWater - A Wonderful LiquidankeetaNo ratings yet
- Observation 1.2Document4 pagesObservation 1.2MARK OLIVER MURILLONo ratings yet
- Benefits of Separating Mixture in Community Grade 6Document15 pagesBenefits of Separating Mixture in Community Grade 6Annabel BallanNo ratings yet
- Module 2 - Water TreatmentDocument56 pagesModule 2 - Water TreatmentGorgeous boiNo ratings yet
- ScienceDocument6 pagesScienceTamuno MatthewNo ratings yet
- Lab - DecantationDocument20 pagesLab - DecantationRaz Mahari100% (9)
- Yoan Salsabilla 1941420016 1A-D4: Pair Up and DiscussDocument3 pagesYoan Salsabilla 1941420016 1A-D4: Pair Up and DiscussShahnaz IsnainiNo ratings yet
- 5 Separatopn of Substances: MixtureDocument7 pages5 Separatopn of Substances: MixtureCris CNo ratings yet
- Contextualized DLP Week 5 Day 4Document7 pagesContextualized DLP Week 5 Day 4Sonny MatiasNo ratings yet
- SPM Chemistry (Form 5) - Chapter 5 - Chemicals For Consumers (Worksheet 03)Document2 pagesSPM Chemistry (Form 5) - Chapter 5 - Chemicals For Consumers (Worksheet 03)Darren TaiNo ratings yet
- Pepsi ReportDocument43 pagesPepsi ReportShoaib ShahidNo ratings yet
- Life As A Scientist: 1. States of MatterDocument5 pagesLife As A Scientist: 1. States of MatterManisha ManishaNo ratings yet
- (W) .ch.6 STD 6 ScienceDocument8 pages(W) .ch.6 STD 6 ScienceSmit JayaniNo ratings yet
- ExperimentaltechniquesDocument52 pagesExperimentaltechniquesDivya Rao100% (1)
- Module 1 Q1 Gen Chem I v.2Document17 pagesModule 1 Q1 Gen Chem I v.2Gweneth BenjaminNo ratings yet
- Sepration of Substances Related Questions (CH 5)Document17 pagesSepration of Substances Related Questions (CH 5)Srijan MauryaNo ratings yet
- Upper Final-Practice Questions - April 2023 Ans KeyDocument14 pagesUpper Final-Practice Questions - April 2023 Ans KeyVi NgôNo ratings yet
- Separation of Substances GR 6 2022-23Document3 pagesSeparation of Substances GR 6 2022-23Prabhu VelNo ratings yet
- Unit 2Document81 pagesUnit 2sethupathy ANo ratings yet
- DecantationDocument7 pagesDecantationMUHAMMAD AKRAMNo ratings yet
- Decatation MethodDocument20 pagesDecatation MethodTuongVNguyenNo ratings yet
- Site VisitDocument13 pagesSite VisitrasanghafarNo ratings yet
- Chapter - 1 Introduction To WTP: 1.1 Potable Water PurificationDocument11 pagesChapter - 1 Introduction To WTP: 1.1 Potable Water PurificationAdesh DeshbhratarNo ratings yet
- CBSE-Cls6-Separation of Substances NotesDocument12 pagesCBSE-Cls6-Separation of Substances NotesPankaj KumarNo ratings yet
- Unit 3Document6 pagesUnit 3sabrina enadaNo ratings yet
- SCIENCEDocument25 pagesSCIENCEcharu.chughNo ratings yet
- Universal Education: Icse Grade ViDocument8 pagesUniversal Education: Icse Grade Vipallavi shindeNo ratings yet
- Report On Sedimentation, Coagulation and Filtration: Name-Section - Semester - Roll NoDocument8 pagesReport On Sedimentation, Coagulation and Filtration: Name-Section - Semester - Roll NoArghyadip SarkarNo ratings yet
- Class 6 WaterDocument7 pagesClass 6 WaterBranded HackerNo ratings yet
- Q1-W5-D5-Separating Mixtures - DistillationDocument4 pagesQ1-W5-D5-Separating Mixtures - DistillationHeidi Dalyagan DulnagonNo ratings yet
- Separation of Substances Notes Grade 6Document5 pagesSeparation of Substances Notes Grade 6Sanaya SinghNo ratings yet
- ChemistryDocument14 pagesChemistryAbhay PantNo ratings yet
- WST Chapter 4 &5 HandoutDocument39 pagesWST Chapter 4 &5 HandoutTadesse MegersaNo ratings yet
- Chapter 5 Water - SolutionDocument14 pagesChapter 5 Water - SolutionREKSHENA A/P PERKAS MoeNo ratings yet
- VVV VVVVV VVV: V#V VVVVV#V V. V VC'V#V &Document3 pagesVVV VVVVV VVV: V#V VVVVV#V V. V VC'V#V &Accu Cii UnixcaNo ratings yet
- Quarter 1 Module 3: Simple Separation Technique: Name: Anya S. Salik Grade and Section: G12-STEM - SisonDocument6 pagesQuarter 1 Module 3: Simple Separation Technique: Name: Anya S. Salik Grade and Section: G12-STEM - SisonNaila SalikNo ratings yet
- Quarter 2-Module 4-Water As Universal SolventDocument13 pagesQuarter 2-Module 4-Water As Universal SolventCes Michaela CadividaNo ratings yet
- Class 7 CHP 18Document3 pagesClass 7 CHP 18GiteshNo ratings yet
- Lastly, We Might Note The Apparent Similarity of This Exercise To The Traditional Exercise ofDocument2 pagesLastly, We Might Note The Apparent Similarity of This Exercise To The Traditional Exercise ofAlifia LuzzaNo ratings yet
- Types of Water SupplyDocument8 pagesTypes of Water SupplyMardiyya SuleimanNo ratings yet
- Class-Vi (Chapter-05) Separation of Substances AnswersDocument2 pagesClass-Vi (Chapter-05) Separation of Substances AnswersMary MorseNo ratings yet
- Water Treatment Process - Objectives & Methods of Water Treatment ProcessDocument5 pagesWater Treatment Process - Objectives & Methods of Water Treatment ProcesstefovNo ratings yet
- First Quarter Module 6: Week 6Document15 pagesFirst Quarter Module 6: Week 6Mariel SalazarNo ratings yet
- Air and Water Copy Work 21-22Document6 pagesAir and Water Copy Work 21-22singh rageeniNo ratings yet
- EvaporationDocument11 pagesEvaporationEduardoAlejoZamoraJr.No ratings yet
- General Chemistry 1 First Quarter - Module 1 Properties of MatterDocument28 pagesGeneral Chemistry 1 First Quarter - Module 1 Properties of MatterJRAVPNo ratings yet
- NameDocument5 pagesNameSemoga MembantuNo ratings yet
- Oil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksFrom EverandOil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksNo ratings yet
- Dps Modern Indian School, Doha, Qatar Dps Modern Indian School, Doha, QatarDocument3 pagesDps Modern Indian School, Doha, Qatar Dps Modern Indian School, Doha, QatarsangopsNo ratings yet
- Class6 SA1 - Class VI - 2014-2015Document4 pagesClass6 SA1 - Class VI - 2014-2015sangopsNo ratings yet
- 2019 AssignmentDocument4 pages2019 AssignmentBNo ratings yet
- Bisect A Line SegmentDocument2 pagesBisect A Line SegmentsangopsNo ratings yet
- Electricity and Circuit Final NotesDocument5 pagesElectricity and Circuit Final NotessangopsNo ratings yet
- River Map of IndiaDocument8 pagesRiver Map of IndiasangopsNo ratings yet
- 3D ShapesDocument2 pages3D ShapessangopsNo ratings yet
- Weekly Test 2 2017 - 18 - Class VI - 2017-2018Document4 pagesWeekly Test 2 2017 - 18 - Class VI - 2017-2018sangopsNo ratings yet
- ENGLISH-Class V-Half Yearly Examination Syllabus 2017-18Document1 pageENGLISH-Class V-Half Yearly Examination Syllabus 2017-18sangopsNo ratings yet
- GENERAL SCIENCE-Class V-Half Yearly Examination Syllabus 2017-18Document1 pageGENERAL SCIENCE-Class V-Half Yearly Examination Syllabus 2017-18sangopsNo ratings yet
- Classes V-X - First Term Examination - Computer Science Syllabus 2018-19 - RevDocument2 pagesClasses V-X - First Term Examination - Computer Science Syllabus 2018-19 - RevsangopsNo ratings yet
- Weekly Test 2 2017 - 18 - Class VI - 2017-2018Document3 pagesWeekly Test 2 2017 - 18 - Class VI - 2017-2018sangopsNo ratings yet
- Assignment Grade 2Document3 pagesAssignment Grade 2sangopsNo ratings yet
- EVS Assignment OctoberDocument4 pagesEVS Assignment OctobersangopsNo ratings yet
- Maths Assignment - Oct 2017Document4 pagesMaths Assignment - Oct 2017sangopsNo ratings yet
- Revision Paper For Class 4Document3 pagesRevision Paper For Class 4sangopsNo ratings yet
- STD 5 - Oct AssignmentDocument7 pagesSTD 5 - Oct AssignmentsangopsNo ratings yet
- AssignmentDocument6 pagesAssignmentsangopsNo ratings yet
- Class 5 Computer Science AssignmentDocument4 pagesClass 5 Computer Science AssignmentsangopsNo ratings yet
- Hot Deserts&Temperate ZoneDocument6 pagesHot Deserts&Temperate ZonesangopsNo ratings yet
- Cleanliness in SchoolsDocument3 pagesCleanliness in SchoolssangopsNo ratings yet
- Dps-Modern Indian School, Alwakrah, Qatar ACADEMIC YEAR (2016-2017) Subject: Social StudiesDocument8 pagesDps-Modern Indian School, Alwakrah, Qatar ACADEMIC YEAR (2016-2017) Subject: Social StudiessangopsNo ratings yet
- Class I - 3rd Weekly Test Syllabus - 2016-17Document3 pagesClass I - 3rd Weekly Test Syllabus - 2016-17sangopsNo ratings yet
- Write Each Number in Prime Factorization Form. 24 39Document2 pagesWrite Each Number in Prime Factorization Form. 24 39sangopsNo ratings yet
- Evs LLM Good MannersDocument3 pagesEvs LLM Good Mannerssangops100% (3)
- Cleanliness in SchoolsDocument3 pagesCleanliness in SchoolssangopsNo ratings yet
- Dps Modern Indian School, Al-Wakra-Qatar: QI. List Any Three Powerful Kings of Mughal Emphire - According To TheDocument5 pagesDps Modern Indian School, Al-Wakra-Qatar: QI. List Any Three Powerful Kings of Mughal Emphire - According To ThesangopsNo ratings yet
- Write Each Number in Prime Factorization Form. 540 172Document2 pagesWrite Each Number in Prime Factorization Form. 540 172sangopsNo ratings yet
- Write Each Number in Prime Factorization Form. 54 35Document2 pagesWrite Each Number in Prime Factorization Form. 54 35sangopsNo ratings yet
- Causes and Effects of Sand ProductionDocument11 pagesCauses and Effects of Sand ProductionSebastian Zarate VilelaNo ratings yet
- Free-Fall Practice ProblemsDocument1 pageFree-Fall Practice ProblemsGabriel Muñiz NegrónNo ratings yet
- On-Demand Manufacturing of Clinical-Quality BiopharmaceuticalsDocument15 pagesOn-Demand Manufacturing of Clinical-Quality BiopharmaceuticalscyannNo ratings yet
- General Notes-Layout1Document1 pageGeneral Notes-Layout1Akmal KhanNo ratings yet
- Lithology and Porosity Determination: Mark of SchlumbergerDocument13 pagesLithology and Porosity Determination: Mark of SchlumbergerSlim.B100% (1)
- Reduction PotentialDocument7 pagesReduction PotentialDharmendra SinghNo ratings yet
- Star Chart June 2022Document1 pageStar Chart June 2022Honolulu Star-AdvertiserNo ratings yet
- Chemical Composition of Lemon Citrus LimDocument5 pagesChemical Composition of Lemon Citrus LimRoberto RebolledoNo ratings yet
- Buffer HPLC PDFDocument1 pageBuffer HPLC PDFSibadattaSenapatiNo ratings yet
- Physics of Atoms and MoleculesDocument7 pagesPhysics of Atoms and MoleculesKAUSTAV DUTTANo ratings yet
- Liquid Nitrogen As A Non Polluting FuelDocument30 pagesLiquid Nitrogen As A Non Polluting FuelShubham Raghuvanshi100% (2)
- Power-Plant Control and Instrumentation BDocument4 pagesPower-Plant Control and Instrumentation BTrustWorthy100No ratings yet
- 4PH1 2PR MSC 20210304Document13 pages4PH1 2PR MSC 20210304Dazy ChowdhuryNo ratings yet
- Structure of The AtomDocument24 pagesStructure of The AtomKunalNo ratings yet
- Physics ProjectileDocument13 pagesPhysics ProjectileAnonymous r9DocC1WNo ratings yet
- Coal Specs Sheet GAR 6400Document2 pagesCoal Specs Sheet GAR 6400Adhitya AchmadNo ratings yet
- An Approach of The Historical Aspects The Advantages and Disadvantages of Automated Analyzers Analysis in Segmented FlowDocument6 pagesAn Approach of The Historical Aspects The Advantages and Disadvantages of Automated Analyzers Analysis in Segmented FlowMiguel Angel Hanco ChoqueNo ratings yet
- Guardian GreaseDocument2 pagesGuardian Greasegacm98No ratings yet
- Mercator's Projection: A Comparative Analysis of Rhumb Lines and Great CirclesDocument36 pagesMercator's Projection: A Comparative Analysis of Rhumb Lines and Great CirclesGeani MihaiNo ratings yet
- Physics 202 Experiment #7 Diffraction Grating Pre-LabDocument6 pagesPhysics 202 Experiment #7 Diffraction Grating Pre-LabDennis LingNo ratings yet
- Chemical Changes and ReactionsDocument8 pagesChemical Changes and ReactionsHarshit KukrejaNo ratings yet
- R05422105 Hypersonic AerodynamicsDocument4 pagesR05422105 Hypersonic AerodynamicsPratap VeerNo ratings yet
- ElektrolisisDocument48 pagesElektrolisisrofiqaasriNo ratings yet
- Guide On Designing A Solar Photovoltaic Powered DC Water PumpDocument6 pagesGuide On Designing A Solar Photovoltaic Powered DC Water PumpDesmondNo ratings yet
- 3 Simple Ways To Calibrate and Use A PH Meter - WikiHowDocument3 pages3 Simple Ways To Calibrate and Use A PH Meter - WikiHowAhmed TaherNo ratings yet