Professional Documents
Culture Documents
United States Patent Office: As The Central Atom On A Carrier
Uploaded by
RasoulOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
United States Patent Office: As The Central Atom On A Carrier
Uploaded by
RasoulCopyright:
Available Formats
United States Patent Office 3,293,280
Patented Dec. 20, 1966
2
able physical deterioration, thereby providing an effica
3,293,280 cious vapor phase catalytic process for the production of
PROCESS FOR THE PREPARATION OF unsaturated nitriles from certain olefins, ammonia and
UNSATURATED NITRLES oxygen, and more especially acrylonitrile from propylene,
Howard S. Young and Edgar L. McDaniel, Kingsport, ammonia and oxygen. The catalyst compositions are
Tenn., assignors to Eastman Kodak Company, Roches especially well adapted for continuous modes of oper
ter, N.Y., a corporation of New Jersey
No Drawing. Fied June 15, 1964, Ser. No. 375,304 ation as, for example, in a fluidized bed type of reactor.
8 Claims. (CI. 260-465.3) It is, accordingly, an object of the invention to pro
vide a novel vapor phase process for the preparation of
This invention relates to a process for preparing un 10 unsaturated aliphatic nitriles, and in particular acrylo
Saturated aliphatic nitriles and to novel catalyst com nitrile from propylene, ammonia and oxygen, by use of a
positions useful in the process of the invention. More catalyst comprising an essentially intimate mixture of an
particularly, it relates to a vapor phase process for the Oxide of arsenic alone or together with an oxide of
production of acrylonitrile by the reaction of propylene, chromium, manganese, iron or boron and a heteropoly
ammonia and oxygen, in the presence of a catalyst com 5 acid of molybdenum containing chromium as the central
prising a mixture of an oxide of arsenic alone, or together atom on a carrier.
with an oxide of chromium, manganese, iron or boron and Another object is to carry out the conversion of propyl
a heteropoly acid of molybdenum containing chromium ene, annonia and oxygen to acrylonitrile in a continuous
as the central atom on a carrier. ac.
Unsaturated aliphatic nitriles have utility in a wide 20 Another object of the invention is to provide a novel
variety of commercial applications. Thus, acrylonitrile catalyst composition for the preparation of unsaturated
is known to be useful as an intermediate in organic aliphatic nitriles, and in particular acrylonitrile from pro
Syntheses of pharmaceuticals, dyes, etc., as well as being pylene, ammonia and oxygen, comprising an essentially
the basic component in many synthetic polymers that are intimate mixture of an oxide of arsenic alone or together
useful for preparing fibers, films, molded articles, and 25 with an oxide of chromium, manganese, iron or boron
the like. It is known that unsaturated aliphatic nitriles and a heteropoly acid of molybdenum containing chromi
can be prepared by reacting olefins with ammonia under um as the central atom on a carrier.
oxidizing conditions at elevated temperatures. For ex Other objects will become apparent from the general
ample, J. N. Cosby in U.S. Patent No. 2,481,826, issued description and examples hereinafter.
September 13, 1949, describes the preparation of lower 30 In accordance with the invention, we prepare unsatu
aliphatic nitriles such as acrylonitrile, methacrylonitrile rated aliphatic nitriles, and more especially acrylonitrile,
and acetonitrile by reacting an olefin such as propene, by passing a feed mixture comprising a short-chain olefin
butene-1, etc. with ammonia and oxygen, at 400-600 C., containing from 3-4 carbon atoms such as, for example,
in the presence of various oxidation catalysts and espe propylene or isobutylene, ammonia and oxygen, in vapor
cially vanadium oxides containing molybdenum oxide. 35 phase at elevated temperatures, over a catalyst comprising
Where propene was used as the starting olefin, yields not an intimate mixture of (1) an oxide of arsenic alone or
exceeding about 6 mole percent (Example 6) of acrylo together with an oxide of chromium, manganese, iron or
nitrile and a substantial amount (10 mole percent) of boron and (2) a heteropoly acid of molybdenum con
hydrogen cyanide were obtained. In J. D. Idol, Jr., U.S. taining chromium as the central atom on a carrier. The
40 reaction is illustrated with the preferred process, namely,
Patent No. 2,904,580, issued September 15, 1959, a
vapor phase method is also described for preparing the conversion of propylene, ammonia and oxygen to
acrylonitrile, wherein a mixture of propylene, ammonia acrylonitrile, as follows:
and oxygen is passed over a catalyst comprising the bis
muth, tin, and antimony salts of phosphomolybdic and 45
molybdic acids and bismuth phosphotungstate. This
process is stated to require not only careful control of A minor proportion of acetonitrile is also formed.
the surface area of the catalyst and pressure conditions, The ratios of the reactants can be varied widely from
but the amount of water employed is a critical factor. the theoretical mole ratios of propylene:oxygen:ammonia
We have now found that by passing a mixture of a 50 of 1:1.5:1. Ratios near this, i.e. about 1: about 1.5:
short-chain olefin, ammonia and oxygen, in certain pro about 1 are preferable; however, the process is operable
portions, at certain elevated temperatures and in vapor at propylene:oxygen ratios from 1:0.1 to 1:5, and propyl
phase, over a catalyst comprising an essentially intimate ene: ammonia ratios from 1:0.1 to 1:5. Both propylene:
mixture of (1) an oxide of arsenic alone or together oxygen and propylene: ammonia ratios may be varied
with an oxide of chromium, manganese, iron or boron 55 from the theoretical ratios.
and (2) a heteropoly acid of molybdenum containing Nitrogen may be fed to reactor. This has no particular
chromium as the central atom on a carrier the reaction effect upon the chemistry involved; but has the practical
goes smoothly to a relatively higher conversion to the advantage that since nitrogen is not detrimental, air
principal product an unsaturated aliphatic nitrile, for may be used as the source of oxygen. In this case, the
example, where the olefin is propylene to acrylonitrile, 60 oxygen to nitrogen ratio is approximately 1:4. Water
and to a considerably lesser amount of acetonitrile, with may be fed to the reactor or it may omitted. It acts as
a minimum of by-products as compared with prior art a diluent and, when used, the preferred amounts in the
processes such as mentioned above, and that water is not form of water vapor range from 0.05-2.0 moles, or more,
critical to efficient operability of this process since excel per mole of propylene in the feed. The temperature of
the reaction can be varied from about 300° C. to about
lent conversions and yields of acrylonitrile can be obtained
without water as the diluent. The reaction products can 65 600° C., but preferably ranges from about 400° C. to
be readily recovered from the effluent stream from the about 550° C. The reaction is not significantly pressure
reactor by conventional means, e.g. by fractional distil dependent. For example, it may be operated satisfactorily
lation of the effluent condensate. at atmospheric pressure, which condition is preferred, but
The catalyst compositions used in carrying out the 70
lower or sub-atmospheric pressures and higher or super
process of the invention retain their activity and selec atmospheric pressures may also be used to give generally
tivity over relatively long-life periods without appreci similarly good results, e.g. from slightly below atmospheric
3,293,280
3. 4.
to about 5 atmospheres. The choice of operating pres by us in carrying out the process of the invention, an
sures may be governed by economic considerations such essentially intimate mixture of a heteropoly acid of molyb
as are involved in plant design, product recovery, etc. denum containing chromium as the central atom such as,
The gaseous hourly space velocity (GHSV) may also be for example, hexamolybdochromic acid having the empiri
varied over a wide range, for example, values (STP) as cal formula HaCrMogO21, a carrier and an oxide of arsenic
low as 100 may be used, and values as high as 6000 may Such as arsenic trioxide (AsO3) or arsenic pentoxide
be used. The preferred space velocity is in the range of (As2O5) or mixtures thereof, and also, if desired, an
about 150 to about 1000. The catalyst may be used either oxide of chromium, maganese, iron or boron or mixtures
in a fixed bed or in fluidized state. In the latter case, thereof, is prepared and calcined. The calcination can
the catalyst exists as small particles which are suspended O be carried out, for example, by heating the catalyst mix
in an upflowing stream of reactant gases. The latter ture at a temperature of from about 200° C. to about
method of carrying out the invention offers advantages 600 C. for a period of several hours or more. The
such as, for example, superior temperature control, and calcined mixture is then reduced to operable granules
less explosive hazard: As previously indicated, water may or particles. Preferably the calcining operation is car
be included, if desired, although this is not critical for the 5 ried out in the presence of air or other suitable oxygen
reaction goes well without such addition. When isobutyl containing gaseous mixture. However, it can be con
ene is substituted for the propylene in the above described ducted in the absence of oxygen.
process, the principal product is methacrylonitrile. The heteropoly acid or its ammonium salt can be used
In general, any type of apparatus that is suitable for in the preparation of the catalyst. Presumably, the am
carrying out the process of the invention in the vapor 20 monium salt of the acid decomposes wholly, or in part,
phase may be employed, e.g., a tubular type of reactor to ammonia and the acid under calcination or during use
or furnace which can be operated in continuous or in at reaction temperature. The concentration of the het
termittent manner and is equipped to contain the catalyst eropoly acid of molybdenum containing chromium can
in intimate contact with the entering feed gases. The vary from about 5% to about 60% (preferably 30 to
reacted gases are then conducted to suitable cooling and 25 50%) by Weight of the catalyst. The concentration of
separatory equipment and the products further separated the oxide of arsenic, calculated as AsOs, can vary from
and recovered by any of the methods known to those about 1% to about 20% (preferably 2 to 9%) by weight
skilled in the art. For example, one such method involves of the catalyst. The concentration of the oxide of chro
scrubbing the effluent gases from the reactor with cooled mium, manganese or iron can vary from about 0.1% to
water or an appropriate solvent to remove the products 30 about 25% (preferably 2 to 10%) by weight of the cata
of the reaction. In such case, the ultimate nitrile products lyst. The concentration of the oxide of boron can vary
may be separated by conventional means such as distill from about 0.1% to a maximum of about 5.0% (prefer.
lation of the resulting liquid mixtures. Unreacted am ably 0.5 to 2%) calculated as boric oxide, by weight of
monia and olefin may be recovered and recirculated the catalyst. The heteropoly acid is always present in
through the system. Spent catalyst may also be reacti 35 a greater percent by Weight than the oxide of arsenic or
vated by heating in contact with air. the oxide of chromium, manganese, iron or boron. The
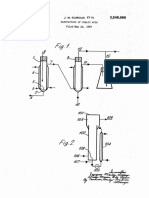
The fluidized bed reactor employed by us in carrying carrier can comprise about 30% to about 94% by weight
out the process of the examples consisted of an upright of the catalyst composition. The most outstanding re
cylindrical tube of Wycor glass of 15 inches length. The Sults in accordance with the invention are obtained with
lower portion of the tube has an internal diameter of 40 40 catalysts comprising 40 to 70% by weight of carrier.
mm. and terminates in a conical bottom which is about 1. The heteropoly acid and the metallic oxides just named
inch in length. The lower portion of the tube including are Supported on a carrier because it is advantageous
the conical bottom is 25 cm. in length. The upper por to Support them on a carrier. The percentages just given
tion of the cylindrical reactor tube has an internal diam- . are for calcined carrier-supported catalysts. Thus the
eter of 55 mm. throughout the greater portion of its 45 weight of the catalyst includes the weight of the carrier.
length and is tapered at its ends, i.e. where it forms the It may be that there is compound formation between
top end of the cylindrical reactor tube and where it joins the oxide of arsenic and the oxide of chromium, man
the portion of the reactor tube which has an internal ganese, iron or boron. The chromium, manganese, iron
diameter of 40 mm. Feed gases were introduced through and boron components can be added directly as oxides or
an inlet tube extending from the top of the reactor and 50 in the form of any other compounds, for example, salts
passing through the center of the reactor into the bottom Such as nitrates, sulfates, etc. which decompose to the
of the catalyst bed. This served to fluidize the catalyst oxides on heating. Compounds of boron such as borates,
bed and to preheat the feed gases. The reactor was metaborates and pyroborates can also be used. Chromic
heated electrically. The effluent gases and vapors were oxide, manganic oxide, ferric oxide and boric oxide are
led from the reactor through a system of traps which 55 the preferred oxides of chromium, manganese, iron and
were cooled with a Dry ice bath. Acrylonitrile, water, boron that can be employed in the novel catalyst compo
acetonitrile, traces of hydrogen cyanide, and unreacted sitions of the invention which are used
the invention. -
in the process of
ammonia were collected in these traps. The condensate
was washed from the thawed traps with water and this Carriers that can be employed, include, for example,
product was analyzed by gas chromatography for nitriles 60 silica, silica-alumina, kieselguhr, pumice, titania, zirconia,
and by titration for ammonia. clay, etc. The use of silica as a carrier is preferred. The
The stripped gas stream was then led through a gas term silica includes silica gel, for example. The cata
sampling valve to a wet test meter. Gas streams were lyst compositions can be readily regenerated by treat
analyzed with Orsat apparatus for unsaturates, carbon ment with air or a gas containing molecular oxygen at or
dioxide, etc. The stripped gas stream generally contained 65 above the reaction temperature. While the arsenic-pro
over 80 percent nitrogen and the rare gases. The un moted molybdenum heteropoly acid catalysts of the in
Saturates as determined by Orsat analysis were shown vention are active and selective for the synthesis, the ad
to be propylene by gas chromatography. The balance dition of the chromium, manganese or iron component
of the gas stream was propylene, oxygen, carbon dioxide, improves the physical strength and activity of the catalyst.
and carbon monoxide. The techniques described were 70 The addition of the boron component improves the ac
found expedient and suitable for the practice of this in tivity of the catalyst. It is possible that when an oxide
vention. Other suitable techniques, such as are known of arsenic promoter is used in conjunction with an oxide
to those skilled in the art, may be used without in any of chromium, manganese or iron, metal arsenates are
way limiting the scope of this invention. formed. Physical strength is important in any solid cata
In preparing the novel catalyst compositions employed lyst, and especially in the case of those to be used in a
3,293,280
5 6
fluidized state where the catalyst must be strong enough hexamolybdochromiate decahydrate. The slurry was
to resist attrition. stirred and heated and set to a gel. A solution of 21.4
The definitions of certain terms used in the examples g. of arsenic pentoxide in 125 ml. of water was added to
and Table 1 are defined as follows: the gel, and the resulting catalyst mixture was stirred and
Contact time is the average time in seconds which the heated. After drying overnight at 120° C., the catalyst
reactants spend at reaction conditions in a volume equal was calcined at 200° C. for four hours. The calcined
to that of the catalyst bed. catalyst obtained was tested as described in Example 2.
Gaseous hourly space velocity (GHSV) is defined as Example 2
the number of volumes of feed gases (STP) which pass
through one volume of catalyst bed in one hour. 0. Two hundred milliliters of 40 x 120 catalyst prepared
The percent conversion to acrylonitrile may be based in Example 1 were charged to the fluidized-solids reactor
on propylene or on ammonia. described hereinbefore. Six runs were made with this
Based on propylene, percent conversion= catalyst using varying conditions. Between runs the cata
lyst was regenerated by fluidizing with air for approxi
moles acrylonitrile formed mately 30 minutes at a temperature of about 430° C. to
moles propylene fed X 100 about 450° C. The runs were of the order of 30 minutes
in length. The mole ratios of CH3:O:NH3:H2O:N
Based on ammonia, percent conversion= were 1:1.5:1:1:6 for each run. Water was used in vapor
moles acrylonitrile formed form.
moles ammonia, fed X 100 20 The feed stream in milliliters per minute, STP, and the
contact time in seconds as well as the gaseous hourly
The yield may be calculated based on propylene or space velocity (GHSV) for runs 1, 2, 3, 4, 5, and 6 are
on ammonia. set forth in Table 1 hereinafter.
TABLE 1.
Run. No Temp., C3H6 NH3 Air Water Contact GEISW
C. Vapor Time
500 200 200 1,500 200 2.0 630
500 200 200 1,500 200 2, O 630
500 267 267 2,000 267 1.5 840
500 143 . 143 1,072 43 2.8 450
480 171 17. 1, 287 17 2.1 540
535 229 229 1,715 229 1.7 720
The results obtained in runs 1, 2, 3, 4, 5, and 6 are set
forth in Table 2 hereinafter.
TABLE 2
Percent
Run No.
Convn. Convn. Yield Yield Conv. Yield
toonACN,
CH6
toonACN,
NH3
of ACN,
on C3H6
ofonACN,
NH3
toonMeCN, of MeCN,
C3H6 on C3H6
35.7 35.7 50.6 35.7 6.3 8.9
38.3 38.3 60.5 38.3 7.9 12.5
33.6 33.6 57.6 33.6 4.7 8.0
33.0 33.0 42.5 33.0 6.2 7. 9
33.6 33.6 49.8 33.6 6.9 0.2
2.1 21. 43.4 2. 6, 8 4.1
ACN stands for acrylonitrile.
Example 3
Based on propylene, percent yield =
A catalyst comprising
moles acrylonitrile formed X 100 55 oxide, 12.1 percent arsenic by weight 8.0 percent chromic
pentoxide, 30 percent hexa
total moles propylene consumed molybdochromic acid and 49.9 percent silica was pre
Based on ammonia, percent yield = pared as follows. 667 g. of an ammonia-stabilized silica
moles acrylonitrile formed
sol which was 30 percent silica were treated with suffi
cient nitric acid to decrease the pH to 6.0. 149 g, of
total moles ammonia, consumed* 100 60 ammonium hexamolybdochromiate were added to the
Conversions and yields to acetonitrile are similarly de silica Sol and the resulting slurry was heated. To the
fined with the moles of acetonitrile formed replacing hot slurry was added a solution of 167 g. of chromic
moles of acrylonitrile in the appropriate expression. nitrate nonahydrate in 200 ml. of water, followed by a
Solution of 48 g. of arsenic pentoxide in 150 ml. of water.
This invention is further illustrated by the following 65 The
Examples 1-4 of preferred embodiments thereof although slurry was dried, and then calcined for 2% hours at
it will be understood that these examples are included 200 C. and 1/2 hours at 500° C. This resulted in a
catalyst with high physical strength.
primarily for purpose of illustration and are not intended The reactor was charged with 150 ml. of 40 x 120 mesh
to limit the scope of the invention unless otherwise of the catalyst prepared in Example 3. A feed stream
specifically indicated. 70 comprising 200 ml. of propylene, 200 ml. of ammonia,
Example 1 1500 ml. of air, and 200 ml. of water vapor per minute,
A catalyst comprising by weight 4.7 percent arsenic STP, was charged to the reactor. The reaction tempera
pentoxide, 39.8 percent hexamolybdochromic acid and ture was 475 C. and the contact time was 1.6 seconds.
55.5 percent silica was prepared as follows. To 840 g. Over 30 minutes of operation, 3.39 g. of acrylonitrile and
of 30 percent silica sol were added 222 g. of ammonium 75 0.41 g. of acetonitrile were recovered. This corresponds
3,293,280
7 3.
to an acrylonitrile conversion of 23.9 percent, based on propylene or isobutylene, respectively, ammonia and oxy
propylene or on ammonia. The yield of acrylonitrile was gen; said mixture containing about 0.1 to about 5 moles of
41.0 percent based on propylene and 25.5 percent based ammonia and about 0.1 to about 5 moles of OXygen per
on ammonia. The conversion to acetonitrile was 3.7 mole of said olefin; in the vapor phase at a temperature
percent. of about 300° C. to about 600 C. in the presence of
Example 4 a solid calcined catalyst which consists essentially of (1)
Example 3 was repeated with the single modification about 1 to about 20 weight percent of arsenic oxide,
that the water vapor used in the feed stream of Example calculated as AsOs, and (2) about 5 to about 60 weight
3 was replaced with nitrogen. Thus, no water vapor percent hexamolybdochromic acid supported on (3) about
was used in this example. The reaction temperature and O 30 to about 94 weight percent of an inert carrier; the
contact time were the same as in Example 3. Over 30 amount of hexamolybdochromic acid being greater than
minutes of operation, 3.81 g. of acrylonitrile and 0.46 g. the amount of arsenic oxide; said catalyst having been
of acetonitrile were produced. This corresponds to an calcined at a temperature of about 200 C. to about
acrylonitrile conversion of 26.8 percent, based on propyl 600 C.
ene or on ammonia. The yield of acrylonitrile was 47.7 5 2. The process of claim 1 in which said solid calcined
percent based on propylene and 28.5 percent based on -catalyst contains about 0.1 to about 25 weight percent
of at least one of
ammonia. The conversion to acetonitrile was 4.1 percent. (a) chromium oxide
In place of the chronic oxide containing catalyst used (b) manganese oxide or
in Examples 3 and 4, there may be substituted generally (c) iron oxide;
similar catalyst compositions wherein the chromic oxide the amount of hexamolybdochromic acid being greater
is replaced with an oxide of manganese. Such as for ex than the amount of said oxide.
ample, MnO, an oxide of iron such as for example, 3. The process of claim 1 in which said solid calcined
FeO, or an oxide of boron such as boric acid, for catalyst contains about 0.1 to about 5 weight percent
example. These catalysts likewise have improved physical 25 boron oxide; the amount of hexamolybdochromic acid be
properties and give generally similar conversions of pro ing greater than the amount of said boron oxide.
pylene to acrylonitrile and isobutylene to methacrylo 4. A process for preparing acrylonitrile or methacrylo
nitrile as the chromic oxide containing catalyst. nitrile which comprises reacting a mixture containing
Other catalyst compositions coming within the speci propylene or isobutylene, respectively, ammonia and oxy
fied ranges of components of the invention can also be 30 gen; said mixture containing about 0.1 to about 5 moles
prepared in accordance with the procedures described of ammonia and about 0.1 to about 5 moles of
hereinbefore. Thus calcined catalytic compositions con oxygen per mole of said olefin; in the vapor phase
taining by weight (a) 10% hexamolybdchromic acid, 2% at a temperature of about 400° C. to about 550° C. in
arsenic pentoxide, 2% chromic oxide and 86% silica; (b) the presence of a solid calcined catalyst which consists
25% hexamolybdochromic acid, 8% arsenic pentoxide, 35 essentially of (1) about 2 to about 13 weight percent of
6% chromic oxide and 61% silica; (c) 35% hexamolyb
dochromic acid, 13% arsenic pentoxide, 10% chromic arsenic oxide, calculated as As2O5, and (2) about 25 to
oxide and 42% silica; (d) 45% hexamolybdochromic about 50 weight percent hexamolybdochromic acid sup
acid, 6% arsenic pentoxide, 15% chromic oxide and 34% ported on (3) about 30 to about 94 weight percent of an
silica; (e) 50% hexamolybdochromic acid, 4% arsenic inert carrier; the amount of hexamolybdochromic acid
40 being greater than the amount of arsenic oxide; said
pentoxide and 46% silica; (f) 60% hexamolybdochromic catalyst having been calcined at a temperature of about
acid, 5% arsenic pentoxide and 35% silica; (g) 40% 200° C. to about 600° C.
hexamolybdochromic acid, 5% arsenic pentoxide, 2% 5. The process of claim 4 in which said solid calcined
manganic oxide and 53% silica; (h) 45% hexamolybdo catalyst contains about 2 to about 10 weight percent of
chromic acid, 5% arsenic pentoxide, 10% manganic oxide 45 at least one of
and 40% silica; (ii) 40% hexamolybdochronic acid, 5% (a) chromium oxide
arsenic pentoxide, 2% ferric oxide and 53% silica; (j) (b) manganese oxide or
35% hexamolybdochromic acid, 4% arsenic pentoxide, (c) iron oxide;
10% ferric oxide and 51% silica; (k) 40% hexamolyb the amount of hexamolybdochromic acid being greater
dochromic acid, 5% arsenic pentoxide and 55% silica; 50 than the amount of said oxide.
(1) 30% hexamolybdochromic acid, 6% arsenic pent 6. The process of claim 4 in which said solid calcined
oxide and 64% silica; (m) 30% hexamolybdochromic catalyst contains about 0.5 to about 2 weight percent
acid, 2% arsenic pentoxide, 2% boric acid and 66% boron oxide; the amount of hexamolybdochromic acid
silica; (n) 40% hexamolybdochromic acid, 5% arsenic being greater than the amount of said boron oxide.
pentoxide, 0.5% boric acid and 54.5% silica; (o) 50% 55 7. The process of claim 4 in which said inert support
hexamolybdochromic acid, 9% arsenic pentoxide, 1% is silica.
boric acid and 40% silica and (p) 25% hexamolybdo 8. The process of claim 4 in which said oxygen is sup
chromic acid, 5% arsenic pentoxide, 2% boric acid and plied as air. .
68% silica, for example, can be prepared and used in the
process of our invention. These catalysts likewise give 60 References (Cited by the Examiner
high conversions of propylene, ammonia, and oxygen to UNITED STATES PATENTS
acrylonitrile with a minimum production of acetonitrile
and other by-products when employed in accordance with 2,606,877 8/1952 West --------------- 252-432
the process of the invention. Also, as previously indi 3,086,041 4/1963 Hadley et al. ------ 260-465.3
cated, isobutylene may be substituted for the propylene 65
3,135,783 6/1964 Sennewald et al. 260-465.3
in the examples to give satisfactory yields of methacrylo 3,142,697 7/1964 Jennings et al. ------ 260-465.3
nitrile. 3,173,957 3/1965 McDaniel et al. 260-465.3 XR
This invention has been described in detail with par 3,200,141 8/1965 Milberger et al. -- 260-465.3
ticular reference to preferred embodiments thereof, but 3,226,241 12/1965 Giordano et al. ---- 260-465.3
it will be understood that variations and modifications FOREIGN PATENTS
can be effected within the spirit and scope of the invention 70
as described hereinbefore and as defined in the appended 1,269,383 7/1961 France.
claims. 957,022 5/1964 Great Britain.
What we claim is:
1. A process for preparing acrylonitrile or methacrylo CHARLES B. PARKER, Primary Examiner.
nitrile which comprises reacting a mixture containing 75 J. P. BRUST, Examiner.
You might also like
- Transition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesFrom EverandTransition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesNo ratings yet
- Process For AADocument15 pagesProcess For AASantiago BorgesNo ratings yet
- Patent US3801634Document5 pagesPatent US3801634Santiago BorgesNo ratings yet
- US3639461Document5 pagesUS3639461M. IDRISNo ratings yet
- United States Patent Office: Nahfeii (Edta) )Document2 pagesUnited States Patent Office: Nahfeii (Edta) )Giacomo AccomandoNo ratings yet
- United States Patent Of?ce: Patented June 17, 1969Document3 pagesUnited States Patent Of?ce: Patented June 17, 1969Rawlinson TolentinoNo ratings yet
- US3093691Document2 pagesUS3093691Ayu GirlsNo ratings yet
- Aliphatic AminesDocument2 pagesAliphatic AminesAmar PandeyNo ratings yet
- Us 3321498Document3 pagesUs 3321498BurakAdayNo ratings yet
- Us 3549696Document4 pagesUs 3549696budispartanNo ratings yet
- US3126422 EnglishDocument3 pagesUS3126422 EnglishMarike Bunga HarfintanaNo ratings yet
- Process Control LDocument23 pagesProcess Control Ltariq fareedNo ratings yet
- The Highly Selective Oxidation of Cyclohexane To Cyclohexanone and Cyclohexanol Over Valpo Berlinite by Oxygen Under Atmospheric PressureDocument9 pagesThe Highly Selective Oxidation of Cyclohexane To Cyclohexanone and Cyclohexanol Over Valpo Berlinite by Oxygen Under Atmospheric PressureFelipe2 HoyosNo ratings yet
- Journal of The American Chemical Society 1950, 72, 5, 1888-1891Document4 pagesJournal of The American Chemical Society 1950, 72, 5, 1888-1891prashantNo ratings yet
- Styrene Methods 2520of ProductionDocument9 pagesStyrene Methods 2520of ProductionMohd Zulazreen50% (2)
- United States Patent (15) 3,697,598: Leif Urban Folke Thorsen, Örn Primary Examiner-Retiri RaymondDocument7 pagesUnited States Patent (15) 3,697,598: Leif Urban Folke Thorsen, Örn Primary Examiner-Retiri RaymondSalsabila CacaNo ratings yet
- A Comparative Study of Non-Oxidative Pyrolysis and Oxidative Cracking of Cyclohexane To Light AlkenesDocument17 pagesA Comparative Study of Non-Oxidative Pyrolysis and Oxidative Cracking of Cyclohexane To Light AlkenesArif HidayatNo ratings yet
- United States Patent Office: Patented Feb. 6, 1951Document3 pagesUnited States Patent Office: Patented Feb. 6, 1951karmilaNo ratings yet
- United States Patent 0: Patented Apr. 13, 1971 2Document3 pagesUnited States Patent 0: Patented Apr. 13, 1971 2NicolHernandezNarvaezNo ratings yet
- Topical and Prospective Processes of Acetoxylation: Grzegorz Lewandowski, Marcin Bartkowiak, Eugeniusz MilchertDocument6 pagesTopical and Prospective Processes of Acetoxylation: Grzegorz Lewandowski, Marcin Bartkowiak, Eugeniusz MilchertAnonymous b9fcR5No ratings yet
- 49 2 Philadelphia 10-04 1181Document4 pages49 2 Philadelphia 10-04 1181lumengentiunNo ratings yet
- United States Patent (19) : 54 Process For Producing AcrylicacidDocument10 pagesUnited States Patent (19) : 54 Process For Producing AcrylicacidKatia Gutierrez GalaNo ratings yet
- A CATALYST: PD/C 1.25 Parts: Effect of Heat TreatmentDocument5 pagesA CATALYST: PD/C 1.25 Parts: Effect of Heat TreatmentFlorian FischerNo ratings yet
- United States Patent 0: Patented Feb. 4, 1969Document3 pagesUnited States Patent 0: Patented Feb. 4, 1969Stella AguirreNo ratings yet
- Reaction TypesDocument10 pagesReaction TypesaqibazizkhanNo ratings yet
- Pines 1968Document9 pagesPines 1968Alejo CastroNo ratings yet
- Light Monoolefins From Methanol And/or Dimethyl Ether: BonifazDocument12 pagesLight Monoolefins From Methanol And/or Dimethyl Ether: BonifazSp4rkZ87No ratings yet
- Degussa Developed Te First Industrial Acrolein Synthesis in The Early 1930sDocument6 pagesDegussa Developed Te First Industrial Acrolein Synthesis in The Early 1930sDasdsa SadadsaNo ratings yet
- Butan-1-Ol and Butan-2-Ol Dehydration On Nitrided Aluminophosphates: Influence of Nitridation On Reaction PathwaysDocument11 pagesButan-1-Ol and Butan-2-Ol Dehydration On Nitrided Aluminophosphates: Influence of Nitridation On Reaction PathwaysDiegowz FiguerzNo ratings yet
- Produksi Phthalic AnhydrideDocument5 pagesProduksi Phthalic Anhydridehalim syarifNo ratings yet
- EOR With Penn State Surfactants: T.G. ArfDocument11 pagesEOR With Penn State Surfactants: T.G. ArfSajad FalahNo ratings yet
- Ulllted States Patent (19) (11) Patent Number: 6,114,278: Karim Et Al. (45) Date 0f Patent: Sep. 5, 2000Document8 pagesUlllted States Patent (19) (11) Patent Number: 6,114,278: Karim Et Al. (45) Date 0f Patent: Sep. 5, 2000federico paNo ratings yet
- A Transient Response Study of The Selective Catalytic Oxidation of Ammonia To Nitrogen On Pt-CuO-Al2O3 Olofsson Et Al Chem. Eng. Sci. 2004Document11 pagesA Transient Response Study of The Selective Catalytic Oxidation of Ammonia To Nitrogen On Pt-CuO-Al2O3 Olofsson Et Al Chem. Eng. Sci. 2004juan davidNo ratings yet
- Decrease in Carbonyl Sulfide in The Feed To Claus Converters Shift CatalystsDocument3 pagesDecrease in Carbonyl Sulfide in The Feed To Claus Converters Shift CatalystsJoel OngNo ratings yet
- Synthesis of Urea From Ammonia and Carbon Dioxide': The Journal Indust'Rial A N D Engineerihtg ChemistryDocument5 pagesSynthesis of Urea From Ammonia and Carbon Dioxide': The Journal Indust'Rial A N D Engineerihtg ChemistryHenry ApNo ratings yet
- Acetone ProductionDocument2 pagesAcetone ProductionpehweihaoNo ratings yet
- Stacks: Ammonia Injection: A Route To CleanDocument8 pagesStacks: Ammonia Injection: A Route To CleanZEN MA100% (1)
- Data Originating From Sources Other Than The EPO May Not Be Accurate, Complete, or Up To DateDocument4 pagesData Originating From Sources Other Than The EPO May Not Be Accurate, Complete, or Up To DateMOHAMMED SHAKEIBNo ratings yet
- United States Patent: Patented Dec. 5, 1967Document6 pagesUnited States Patent: Patented Dec. 5, 1967Attila TamasNo ratings yet
- Us2503724 - Ca2941105a1Document8 pagesUs2503724 - Ca2941105a1Facundo MendezNo ratings yet
- United States Patent 0 ": Patented August 23, 1966Document3 pagesUnited States Patent 0 ": Patented August 23, 1966Citra Adelina SitorusNo ratings yet
- A. Belchetz: Production of Carbon DisulfideDocument7 pagesA. Belchetz: Production of Carbon DisulfidesyafiraNo ratings yet
- Production of Ammonia: Department of Chemical Engineering A Report OnDocument29 pagesProduction of Ammonia: Department of Chemical Engineering A Report OnDr.AhmedNo ratings yet
- Chapter - 2 Process DescriptionDocument11 pagesChapter - 2 Process DescriptionSomak SahujiNo ratings yet
- Nitric Acid PDFDocument12 pagesNitric Acid PDFEhb90210No ratings yet
- Production of AmmoniaDocument29 pagesProduction of AmmoniaBhavna Bajpai83% (6)
- US PatentDocument4 pagesUS Patentaldo BMCNo ratings yet
- Tempo Reagent MechanismDocument27 pagesTempo Reagent MechanismMd Abdullah Al NomanNo ratings yet
- PDDocument2 pagesPDnur_ika_1No ratings yet
- K2SO4 Production Via The Double Decomposition Reaction of KCL and PhosphogypsumDocument11 pagesK2SO4 Production Via The Double Decomposition Reaction of KCL and PhosphogypsumGeorge Van Bommel100% (2)
- Jozmim: Feb. 17, 1953 B. V. Aller EtalDocument7 pagesJozmim: Feb. 17, 1953 B. V. Aller EtalFatonaRifkyPNo ratings yet
- Integrated Process For The Production of Vinyl Acetate From Acetic Acid Via Ethyl AcetateDocument44 pagesIntegrated Process For The Production of Vinyl Acetate From Acetic Acid Via Ethyl Acetatearif ihwandaNo ratings yet
- MetoxidoDocument8 pagesMetoxidocessavelinoNo ratings yet
- Sol-Gel Synthesis of Alumina Using Inorganic Salt PrecursorDocument6 pagesSol-Gel Synthesis of Alumina Using Inorganic Salt PrecursordgslkbmalkdNo ratings yet
- Anhydride ReactionsDocument15 pagesAnhydride ReactionstechkasambaNo ratings yet
- Introduction To AmmoniaDocument15 pagesIntroduction To AmmoniaHameed Akhtar100% (1)
- Sample Preparation For Accelerator-Based Radiocarbon DatingDocument5 pagesSample Preparation For Accelerator-Based Radiocarbon DatingEdwin Angel Silva de la RocaNo ratings yet
- United States Patent 0: Patented Oct. 11, 1966Document4 pagesUnited States Patent 0: Patented Oct. 11, 1966MeilyaniNo ratings yet
- (Clinical Cancer Monographs) John W. L. Fielding MD, FRCS, Jean Powell BSC, FIS, William H. Allum MD, FRCS, John A. H. Waterhouse MA, PHD, HonFFOM, Christopher C. McConkey BSC (Auth.) - Cancer of TheDocument260 pages(Clinical Cancer Monographs) John W. L. Fielding MD, FRCS, Jean Powell BSC, FIS, William H. Allum MD, FRCS, John A. H. Waterhouse MA, PHD, HonFFOM, Christopher C. McConkey BSC (Auth.) - Cancer of TheRasoulNo ratings yet
- نظام السيريلاتDocument1 pageنظام السيريلاتRasoulNo ratings yet
- Trading Hub 3.oDocument36 pagesTrading Hub 3.oDream Rich94% (48)
- Alarming Update On Incidence of Crimean-Congo Hemorrhagic Fever in Iraq in 2023Document6 pagesAlarming Update On Incidence of Crimean-Congo Hemorrhagic Fever in Iraq in 2023RasoulNo ratings yet
- Gastric CancerDocument126 pagesGastric Cancermwani775100% (1)
- AnemiaDocument63 pagesAnemiaRasoulNo ratings yet
- Research Productivity of India and Iran in StomachDocument14 pagesResearch Productivity of India and Iran in StomachRasoulNo ratings yet
- Alarming Update On Incidence of Crimean-Congo Hemorrhagic Fever in Iraq in 2023Document6 pagesAlarming Update On Incidence of Crimean-Congo Hemorrhagic Fever in Iraq in 2023RasoulNo ratings yet
- ALE's Iraq Branch Awarded Karbala Refinery Project ContractsDocument5 pagesALE's Iraq Branch Awarded Karbala Refinery Project ContractsRasoulNo ratings yet
- ZCS05 ZolfyGraduationPrfojectHandbooktemp1Document19 pagesZCS05 ZolfyGraduationPrfojectHandbooktemp1RasoulNo ratings yet
- INTEGRATED MATERNAL NEWBORN and CHILD HEALTHDocument2 pagesINTEGRATED MATERNAL NEWBORN and CHILD HEALTHRasoulNo ratings yet
- Project 2Document61 pagesProject 2Venky Krish BellamkondaNo ratings yet
- Lab3 MicrobiologyDocument4 pagesLab3 MicrobiologyRasoulNo ratings yet
- Karbala Refinery Capacity 140 000 BPSDDocument11 pagesKarbala Refinery Capacity 140 000 BPSDRasoulNo ratings yet
- Men in An PlantDocument13 pagesMen in An PlantJoanna JimenezNo ratings yet
- Kar Bala Refiner y Capacity 140 000 BPSDDocument6 pagesKar Bala Refiner y Capacity 140 000 BPSDRasoulNo ratings yet
- Handbook of Vapor Pressure Volume 4 Inorganic Compounds and Elements Library of Physico Chemical Property DataDocument375 pagesHandbook of Vapor Pressure Volume 4 Inorganic Compounds and Elements Library of Physico Chemical Property DataRasoulNo ratings yet
- Acetic Acid PrintDocument83 pagesAcetic Acid PrintRasoulNo ratings yet
- ZCS05 ZolfyGraduationPrfojectHandbooktemp1Document19 pagesZCS05 ZolfyGraduationPrfojectHandbooktemp1RasoulNo ratings yet
- ALE's Iraq Branch Awarded Karbala Refinery Project ContractsDocument2 pagesALE's Iraq Branch Awarded Karbala Refinery Project ContractsRasoulNo ratings yet
- White PaperDocument24 pagesWhite PaperRasoulNo ratings yet
- RSC Advances: PaperDocument12 pagesRSC Advances: PaperRasoulNo ratings yet
- New Microsoft Word DocumentDocument4 pagesNew Microsoft Word DocumentRasoulNo ratings yet
- Cyano Compounds, Inorganic: 1. Hydrogen CyanideDocument38 pagesCyano Compounds, Inorganic: 1. Hydrogen CyanideRasoulNo ratings yet
- Thermal Gasification of Rice Husks From Rice Growing Areas in Mwea, Embu County, KenyaDocument7 pagesThermal Gasification of Rice Husks From Rice Growing Areas in Mwea, Embu County, KenyaRasoulNo ratings yet
- ALE's Iraq Branch Awarded Karbala Refinery Project ContractsDocument2 pagesALE's Iraq Branch Awarded Karbala Refinery Project ContractsRasoulNo ratings yet
- PMR v40 I4 174 174Document1 pagePMR v40 I4 174 174RasoulNo ratings yet
- Us 2621204Document4 pagesUs 2621204RasoulNo ratings yet
- 2 5364059534434239298Document182 pages2 5364059534434239298RasoulNo ratings yet
- Oil Analysis ReviewDocument44 pagesOil Analysis ReviewFahmi NurfaNo ratings yet
- (Woodhead Publishing India in Textiles) Har Bhajan Singh - Kumar, Avinash Bharati-Handbook of Natural Dyes and Pigments-Woodhead Publishing India PVT LTD (2014)Document306 pages(Woodhead Publishing India in Textiles) Har Bhajan Singh - Kumar, Avinash Bharati-Handbook of Natural Dyes and Pigments-Woodhead Publishing India PVT LTD (2014)Milena Katarina Stojiljkovic0% (1)
- Peenya Industrial Area PDFDocument27 pagesPeenya Industrial Area PDFShashank SundiNo ratings yet
- Company Presentation: AIA Engineering LimitedDocument26 pagesCompany Presentation: AIA Engineering LimitedAl Amin Hossain SrabonNo ratings yet
- Content ServerDocument3 pagesContent ServerLINA KATHERIN ESPINOSA OSPINANo ratings yet
- Taninos Como Tecnologia Limpia CurtiembreDocument18 pagesTaninos Como Tecnologia Limpia CurtiembreAlfredo Quispe LujanoNo ratings yet
- RPG Engineering ProjectDocument58 pagesRPG Engineering ProjectkavyanayakNo ratings yet
- CBSE QUESTION BANK D and F Block ElementsDocument2 pagesCBSE QUESTION BANK D and F Block ElementsVishnuNo ratings yet
- Chemical ChartDocument6 pagesChemical ChartAnurag SarkarNo ratings yet
- 55901413Document248 pages55901413Raushan KumarNo ratings yet
- BOMcheck REACH Candidate List Screening For Supplied Articles - June 2023Document80 pagesBOMcheck REACH Candidate List Screening For Supplied Articles - June 2023ansu.ggmNo ratings yet
- Annual Report 2Document8 pagesAnnual Report 2swayambhu000No ratings yet
- Symbols and Charges For Monoatomic IonsDocument2 pagesSymbols and Charges For Monoatomic IonsaNo ratings yet
- ,MMNBGDocument6 pages,MMNBGAnonymous FW5PVUp100% (1)
- Corrosion Failures of AISI Type 304 Stainless Steel in A Fertiliser PlantDocument11 pagesCorrosion Failures of AISI Type 304 Stainless Steel in A Fertiliser PlantAndrea CalderaNo ratings yet
- 14 00247 (En)Document6 pages14 00247 (En)cccc80cccccNo ratings yet
- Engineering Alloys (Ferrous)Document103 pagesEngineering Alloys (Ferrous)Sukhwinder Singh GillNo ratings yet
- PT Centa Brasindo Abadi-Aircol MR 46Document2 pagesPT Centa Brasindo Abadi-Aircol MR 46azmiazfarNo ratings yet
- Tatin CelikDocument52 pagesTatin Celikgoran073No ratings yet
- Preview - AWS D14.8M-2009 (ISO TR 17844-2004 IDT) PDFDocument8 pagesPreview - AWS D14.8M-2009 (ISO TR 17844-2004 IDT) PDFeduardo Salazar RiveraNo ratings yet
- CHM 477 Experiment 3 4 5 PDFDocument10 pagesCHM 477 Experiment 3 4 5 PDFAhmad ZakwanNo ratings yet
- D-Block Elements WorkbookDocument42 pagesD-Block Elements WorkbookStudy BuddyNo ratings yet
- CHM 101 CorrectedDocument23 pagesCHM 101 CorrectedPeace TemitayoNo ratings yet
- The D and F Block ElementsDocument2 pagesThe D and F Block ElementsnidalNo ratings yet
- What Is CRS Steel and Bar Bending Schedule - QuoraDocument5 pagesWhat Is CRS Steel and Bar Bending Schedule - QuoraPritha DasNo ratings yet
- Trakhees Waste Water Discharge Quality Standard PDFDocument18 pagesTrakhees Waste Water Discharge Quality Standard PDFWaseem SiddiqueNo ratings yet
- 0620 Y16 SP 4Document20 pages0620 Y16 SP 4sookchinNo ratings yet
- Wire Solutions CatalogDocument8 pagesWire Solutions Catalogpumud15No ratings yet
- STAR 3 in 1.1 F Effect of Mineralizers in Cement ProductionDocument25 pagesSTAR 3 in 1.1 F Effect of Mineralizers in Cement Productionthanhtbk2000No ratings yet
- CR39W (HPNB)Document2 pagesCR39W (HPNB)Jean-Noël LerouxNo ratings yet
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Water-Based Paint Formulations, Vol. 3From EverandWater-Based Paint Formulations, Vol. 3Rating: 4.5 out of 5 stars4.5/5 (6)
- Transformer: The Deep Chemistry of Life and DeathFrom EverandTransformer: The Deep Chemistry of Life and DeathRating: 4.5 out of 5 stars4.5/5 (13)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)