Professional Documents
Culture Documents
Serologic Diagnosis of Tuberculosis Using A Simple Commercial Multiantigen Assay
Uploaded by
TanveerOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Serologic Diagnosis of Tuberculosis Using A Simple Commercial Multiantigen Assay
Uploaded by
TanveerCopyright:
Available Formats
Serologic Diagnosis of Tuberculosis
Using a Simple Commercial
Multiantigen Assay*
Mark D. Perkins, MD; Marcus B. Conde, MD; Marneili Martins, MD;
Afranio L. Kritski, MD, PhD
Setting: Seven primary health clinics and a pulmonary disease specialty clinic in Rio de Janeiro
City, Brazil.
Objective: To evaluate a commercial immunochromatographic test kit (ICT Tuberculosis;
AMRAD-ICT; Sidney, Australia) employing five recombinant Mycobacterium tuberculosis pro-
teins for the detection of pulmonary tuberculosis (TB).
Design: Serology test results were compared with duplicate sputum microscopy and culture in 277
patients with symptomatic pulmonary disease (243 with pulmonary TB and 34 with nontubercu-
lous disease). An additional 110 healthy control subjects were also tested.
Results: The serology test was simple and rapid to perform and detected 64.2% of smear-positive
and 46.3% of smear-negative TB patients overall. HIV co-infection was present in 15.3% of TB
patients, and serology was much less sensitive (overall 27.6%) in this small group, as was
microscopy (13.8%). Specificity of the serology test was 100% in healthy control subjects and
85.2% in the small number of control patients with pulmonary disease, including those with prior
TB. Combined with microscopy, serology detected 72.8% of TB patients.
Conclusion: Depending on the population studied, multiantigen serologic tests for TB may be as
sensitive as microscopy, but detect a different and overlapping subset of patients. The use of
multiple antigens in this kit increased test sensitivity without significant loss of specificity. Bacille
Calmette-Guérin vaccination and tuberculin sensitivity did not affect serology results. Estimating
specificity in clinical use will require testing a much larger cohort of symptomatic patients with
nontuberculous disease. The TB diagnostic performance of this group of antigens in HIV
co-infected individuals was poor. (CHEST 2003; 123:107–112)
Key words: developing countries; diagnosis; serology; tuberculosis
Abbreviations: AFB ⫽ acid-fast bacilli; BCG ⫽ bacille Calmette-Guérin; CI ⫽ confidence interval; PPD ⫽ purified
protein derivative; TB ⫽ tuberculosis
T hecost-effective
treatment of tuberculosis (TB) is a highly
medical intervention. Selection of
world laboratories. Many attempts have been made
to develop a serologic TB test, challenges to which
patients for treatment, however, remains compli- include the need to discriminate active from latent
cated by the lack of specificity of clinical findings and infection, to avoid cross-reactivity to bacille
the poor performance characteristics of diagnostic Calmette-Guérin (BCG) or nontuberculous myco-
methods available in the majority of developing- bacteria, and to perform consistently in genetically
and immunologically diverse populations.
Early studies using partially purified antigens
*From the Department of Medicine (Dr. Perkins), Duke Uni-
versity Medical Center, Durham, NC; and Tuberculosis Re- showed that antimycobacterial antibody was readily
search Unit (Drs. Conde, Martins, and Kritski), Chest Service, detected in tuberculosis patients, but test specificity
Clementino Fraga Filho Hospital, Federal University of Rio de
Janeiro, Rio de Janeiro, Brazil. was poor.1,2 Monoclonal antibodies and highly puri-
The study received partial financial support from AMRAD ICT; fied native or recombinant antigens have improved
CNPq-processo (35 04 77/95. 7), NIH Fogarty AIDS Interna- specificity, but at a cost of decreased sensitivity.
tional Training Grant (5 D43 TW00018), and Fogarty Interna-
tional Center (TBITRP 5 D43 TW00018). Recently, the degree to which the humoral response
Manuscript received November 28, 2000; revision accepted July to TB is heterotypic has received greater recognition,
12, 2002. stimulating the development of serology tests using
Correspondence to: Mark D. Perkins, MD, World Health Orga-
nization, Tropical Diseases Research (TDR), 20 Appia Ave, multiple antigens.3
CH-1211 Geneva 27, Switzerland; e-mail: perkinsm@who.int Though sensitivity, specificity, and cost are the
www.chestjournal.org CHEST / 123 / 1 / JANUARY, 2003 107
Downloaded From: http://journal.publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21987/ on 04/20/2017
characteristics most frequently considered in TB Serology
diagnostic tests, robustness and speed are also criti- From case patients, serum for testing was drawn at the time of
cal to field effectiveness. Delays of even 1 to 2 days diagnosis in all but one patient, who was tested 6 months after
result in loss of patients to follow-up, increased cost initial presentation. Testing was done using a commercial kit
from return visits, and diminished physician control (ICT Tuberculosis; AMRAD-ICT; Sydney, Australia) consisting
and test confidence.4,5 A diagnostic tool for TB that of a folding cardboard device containing a nitrocellulose strip
onto which the 38-kd antigen and four other recombinant
could be performed rapidly in the field would be of antigens were fixed in discrete lines. The construction of the
great benefit to tuberculosis control programs, espe- device and its use has been described elsewhere in detail.7
cially in developing countries, even if test perfor- Briefly, 30 L of serum was placed at one end of the nitrocel-
mance were less than ideal. In this report, we lulose strip and allowed to migrate across four bands containing
evaluate a rapid immunochromatographic test kit to the five fixed antigens. After the serum had reached a marked
limit line, migration was stopped with buffer, and captured
detect antibody directed against any one of five antibody detected using an immunogold goat anti-human IgG
recombinant antigens in TB patients in Rio de conjugate. Testing required 5 to 15 min, and ease of use was such
Janeiro City, Brazil. Rio de Janeiro, the fourth- that multiple tests could be run concurrently. Interpretation was
largest city in Latin America, is an evolving mega-city performed as suggested by the manufacturer, and a visible line at
with an expanding epidemic of TB. There were any of the four antigen-binding areas was considered positive.
Each positive band was scored for location (A through D) and
10,210 cases of TB registered in the city in 1997, and intensity of staining (1⫹ to 4⫹). A single investigator read all
a incidence rate based on passive case finding of 120 strips. Serum was frozen, not fresh, and went through at least one
per 100,000 population citywide.6 freeze-thaw cycle prior to use in this test.
Materials and Methods Results
Patient Enrollment Adequate clinical and laboratory information was
available to evaluate 393 of the 494 patients from
Sera were collected between July 1996 and December 1996 whom serum was collected. In six subjects (1.5%),
from three populations of patients not receiving antituberculous kits did not yield readable results, either because of
therapy: (1) patients with newly diagnosed TB at seven Rio de
Janeiro City primary health clinics; (2) patients with pulmonary operator error or because of viscous serum that
disease of indeterminate cause presenting for evaluation by failed to migrate on the nitrocellulose strip. The
induced sputum and/or BAL at Clementino Fraga Filho Hospital, remaining 387 tests formed the basis for our evalu-
Federal University of Rio de Janeiro; and (3) healthy subjects, ation.
including asymptomatic household TB contacts and purified Patients were classified into four groups for anal-
protein derivative (PPD)-positive and PPD-negative health-care
workers at the study site. The control group was thus composed ysis, depending on the results of clinical and labora-
of both asymptomatic volunteers and patients with pulmonary tory findings: (1) patients with smear-positive TB,
disease in whom Mycobacterium tuberculosis was not detected (2) patients with smear-negative TB with or without
on mycobacterial culture or microscopic examination of Ziehl- positive mycobacterial culture, (3) patients with
Neelsen–stained sputum smear. BCG vaccination history was not symptomatic pulmonary disease and no evidence of
recorded, but the age of the patients and the history of universal
BCG vaccination of infants in Brazil predict that between 50% TB, and (4) healthy individuals who were hospital
and 75% would have received the vaccine. workers or household contacts of pulmonary TB
patients.
Case patients had a mean age of 36.0 years,
Exclusions
whereas healthy control subjects tended to be
Symptomatic patients for whom no smear or culture data were younger (29.8 years) and patients with non-TB pul-
available or for whom relevant clinical information was lacking monary disease older (55.4 years). The proportion of
were excluded from analysis. A small number of samples that did men (n ⫽ 158) and women (n ⫽ 85) among the case
not yield test results for technical reasons were also excluded.
patients reflected that routinely reported by the
Brazilian National Tuberculosis Program. HIV test
Clinical and Laboratory Evaluation results were available from 189 of 243 case patients,
All case patients were interviewed and underwent physical and of which 29 were positive (15.3%), and from 28 of
laboratory evaluation including a chest radiograph. Two sputum the 34 control subjects with nontuberculous pulmo-
samples from each patient were submitted for microscopic nary disease, of which none were positive. HIV
examination for acid-fast bacilli (AFB) and culture on Löwen- testing was not performed on healthy control sub-
stein-Jensen media following standard alkaline decontamination. jects.
Smear and culture results were considered positive if either of
the two duplicate samples were positive. HIV testing was per- The results of the ICT Tuberculosis test kit are
formed whenever possible. Asymptomatic control patients under- shown in Table 1. Overall, 55.1% of TB patients
went no laboratory evaluation other than tuberculin skin testing. were test positive with detectable antibodies to one
108 Clinical Investigations
Downloaded From: http://journal.publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21987/ on 04/20/2017
Table 1—TB Test Result in Each Study Group and Antibody Detection by Test Antigen Line
% Positive by Individual Antigen Line
Positive Test Result to Any Antigen,
Patient Group Tested No. (%; 95% CI) Line A Line B Line C Line D
All TB patients (n ⫽ 243) 134 (55.1; 48.8–61.4)
Smear-positive TB (n ⫽ 120) 77 (64.2*; 55.6–72.8) 42.5 10.8 20.0 27.5
Smear-negative TB (n ⫽ 123) 57 (46.3*; 37.5–55.1) 28.5 9.8 16.3 13.8
All non-TB patients (n ⫽ 144) 5 (3.5; 0.5–6.5)
Non-TB pulmonary disease (n ⫽ 34)† 5 (14.8; 2.9–26.7) 5.9 2.9 5.9 8.8
Healthy control subjects (n ⫽ 110)‡ 00
*For test-positive proportion in smear-positive and smear-negative patients (p ⫽ 0.005).
†Including prior TB.
‡Including PPD-positive and PPD-negative subjects.
or more of the five recombinant Mycobacterium the subset with symptomatic pulmonary disease.
tuberculosis antigens in the test. Smear-positive TB Three of these patients had a history of prior TB.
patients were more frequently test positive (64.2%, Two additional symptomatic control patients with no
p ⫽ 0.005), but nearly half of the smear-negative history of prior TB were also seropositive using the
patients (46.3%) were also detected by the kit, test kit. One was a patient with bilateral interstitial
including 50% of the 38 smear-negative, culture- lung disease, and the other was a patient with COPD
negative patients with diagnoses made on clinical and lung cancer of unknown type who died within a
and radiographic grounds. The duration of symp- few months of presentation. No autopsies were
toms ranged from 1 to 58 weeks (average 10.4 weeks) performed. The test kit results from all five of these
in 49 of 243 TB patients for whom firm dates were sera classified as false-positive showed either 3⫹ or
available. Duration of symptoms in patients with stronger scoring on a single band or had multiple
positive serology results was not significantly longer positive bands. Of 113 control subjects who had skin
than in those with negative results (11.5 weeks vs 8.4 tests performed, 23 subjects (20.3%) had positive
weeks, p ⫽ 0.28). tuberculin reactions, none of whom had positive
Band A in the kit used for this study contained the serology test results.
38 kd antigen. The identity of the other four antigens The results of smear, culture, and serology tests
is proprietary. As shown in Table 1, the most com- for tuberculosis case patients, divided by HIV-
monly detected antibody in case patients was that infection status, are shown on Table 2. All patients
directed against the 38-kd antigen, but antibody to submitted two or more sputum specimens for exam-
the other test antigens was present in roughly 10 to ination; AFB microscopy and culture were each
30% of patients. The addition of antigens beyond the considered positive if any one of the respective
38-kd antigen increased sensitivity of the assay 51% examinations were positive. Laboratory results from
in smear-positive patients and 63% in smear- 54 TB patients for whom HIV results were unavail-
negative patients, without markedly diminishing able were not statistically different from those from
specificity. There were an insufficient number of HIV-negative TB patients (p ⬎ 0.5 for all tests), and
false-positive test results to evaluate the impact of these results were pooled for analysis into a single
multiple antigens on specificity. HIV-uninfected group. Among the 214 HIV-
The serology test result was positive in 5 of the 144 uninfected TB patients, microscopy was positive in
sera from control patients, all of whom were from 54.2%, culture in 79.0%, and serology in 58.4%.
Table 2—Results of Duplicate Microscopy (AFB) and Culture and of TB Kit in All TB Patients,
Divided by HIV-Infection Status*
HIV Status TB, No. Positive AFB† Positive Culture† Positive Serology Positive AFB or Serology
Infected 29 13.8 (1.2–26.4) 69.0 (52.2–85.8) 27.6 (11.3–43.9) 37.9 (20.2–55.6)
Uninfected‡ 214 54.2 (47.5–60.9) 79.0 (73.5–84.5) 58.4 (51.8–65.0) 77.1 (71.5–82.7)
Overall 243 49.4 (43.1–55.7) 77.8 (72.6–83.0) 55.1 (48.8–61.4) 72.4 (66.8–78.0)
*Values shown as percentage of test-positive patients (95% CIs).
†Smear and culture considered positive if either of the two samples were positive.
‡Includes 54 patients without HIV test result. Microbiology and serology results from untested group was not statistically different from those of
uninfected (p ⬎ 0.5 for all tests).
www.chestjournal.org CHEST / 123 / 1 / JANUARY, 2003 109
Downloaded From: http://journal.publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21987/ on 04/20/2017
Among the 29 HIV-infected TB patients, microscopy phenotype, and tests designed to detect responses to
was positive in 13.8%, culture in 69.0%, and serology a single antigen could show important geographic
in 27.6%, but confidence intervals (CIs) were wide. variability and limited sensitivity.18 –21 To overcome
CD4 counts were not available on enough of these these problems, multiantigen tests have been devel-
HIV-infected patients to evaluate any association oped.22–24 Loss of specificity is additive, however, so
between the degree of immunosuppression and the each of the antigens used must be very highly
likelihood of false-negative serology results. Combin- specific.
ing acid-fast sputum smear microscopy with serology The test kit used in this study employed five
yielded sensitivity of 77.1% in HIV-uninfected TB different protein antigens to detect an IgG response
patients, which was equivalent to culture (p ⫽ 0.80). to M tuberculosis. Each individual antigen showing a
Combined sensitivity of microscopy plus serology specificity of at least 98% (differences not statistically
was 37.9% in HIV-coinfected patients and 72.4%
significant), resulting in an overall specificity of
overall.
96.5% in the study group. However, this result is
relatively arbitrary, as antibody was detected in none
of the healthy control subjects, and simply increasing
Discussion their number would increase the reported specificity
The need for improved TB diagnostics has long of the test. Of the 34 patients with nontuberculous
been recognized. Local diagnostic needs may vary pulmonary disease included here, 5 patients (14.7%)
markedly, and it is unlikely that a single diagnostic had a positive serology test result, but because of the
test be ideal for all situations. However, an accurate, small size of this group, CIs were wide (2.9 to 26.7).
simple, rapid, point-of-care assay would be widely Symptomatic, culture-negative patients in endemic
useful and could be expected to improve case hold- areas may often have positive results of nucleic acid
ing, empower health-care workers at the peripheral amplification assays25 or serology tests for TB, as was
level, and decrease diagnostic confusion and delay. found in studies of a previous version of the ICT
Recognizing these needs, serologic approaches to Tuberculosis kit.7,26 Determining the true specificity
TB diagnosis have been tried on and off since the of nonculture-based tests in symptomatic patients
seroagglutination work of Arloing8 in 1898. Early TB will require evaluation of patients from nonendemic
serology tests used PPD or other crude antigens and sites and prolonged and careful follow-up of study
showed relatively poor specificity, as many of the patients in disease endemic countries.
dominant antibody responses are to shared anti- The performance characteristics of diagnostic tests
gens.9 –12 for TB vary widely with the population studied. The
The availability of monoclonal antibodies, recom- poor performance of microscopy in HIV-coinfected
binant antigens, and methods for the accurate appli- patients in this study, for example, was due in part to
cation of controlled amounts of immunologic re- an enrollment bias with the purposeful inclusion of
agents to nitrocellulose or other solid matrix have smear-negative HIV-infected patients with pulmo-
enhanced the specificity and reproducibility of such nary disease undiagnosed at initial evaluation. Dif-
tests.13 The best studied M tuberculosis diagnostic ferences in patient populations studied may also
antigen is the 38-kd antigen, and its availability as a account for the higher sensitivity found in previous
recombinant expressed in Escherichia coli led to the trials of a serology test using the 38-kd antigen alone
development of tests that could easily distinguish in a similar format to that employed here. Two
between healthy PPD-positive controls and tubercu- studies of a single antigen ICT Tuberculosis kit
lous patients.14 –16 Development of immunochro- carried out in China demonstrated 70 to 90% sensi-
matographic strips has put these tests into a form tivity in smear-negative and smear-positive patients,
appropriate for disease endemic countries, though respectively.7,26 Greater duration or severity of ill-
the change in format from enzyme-linked immu- ness, which have been correlated with likelihood of
nosorbent assay has sometimes been accompanied positive serologic test for TB, may be one explana-
by a loss of favorable operating characteristics.17 A tion. In the current study, duration of illness did not
number of simple serologic assays using highly puri- correlate with test results in the group from which
fied or recombinant proteins are now being mar- such data were available. Comparison with other
keted in the developing world, many based on published studies of the ICT Tuberculosis test is
detection of antibody to 38 kd, but limited data exist confounded by limitations in the available data. For
to support their use. example, a study in California performed with the
Variations in specific antibody responses to myco- same multiantigen kit used in the present trial found
bacterial antigens in different human populations only 20% sensitivity in a small number (n ⫽ 59) of
may be linked to human leukocyte antigen-DR culture-positive TB cases.27 The lack of supporting
110 Clinical Investigations
Downloaded From: http://journal.publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21987/ on 04/20/2017
clinical and microbiologic data, including an absence nator; Dr. Oscar Berro, Director of Rio de Janeiro State Refer-
of smear microscopy results, make that study diffi- ence Laboratory-LACEN; and the health-care workers from the
seven Primary Health Services in Rio de Janeiro City.
cult to interpret.
There were a number of limitations to the current
study. The first is that the serology testing was done
retrospectively, using stored frozen sera that had References
passed through at least one freeze-thaw cycle. The 1 Daniel T. Antibody and antigen detection for the immunodi-
use of fresh serum may increase sensitivity. A second agnosis of tuberculosis: why not? What more is needed?
limitation to the study is the lack of inclusion of Where do we stand today? J Infect Dis 1988; 158:678 – 680
2 Grange JM, Lazlo A. Serodiagnostic tests for tuberculosis: a
patients with known nontuberculous mycobacterial
need for assessment of their operational predictive accuracy
infections, as cross-reactive antibodies to antigens and acceptability. Bull World Health Organ 1990; 68:571–576
from other mycobacterial species could result in loss 3 Genaro M. Tests to replace AFB microscopy in the diagnosis
of specificity as mentioned by others.28 Thirdly, of smear positive pulmonary diagnosis. WHO Tuberculosis
patients from a single geographic region were in- Diagnostics Workshop. Geneva, Switzerland: World Health
cluded, limiting the certainty that these data apply to Organization, 1997
other regions. Lastly, the study enrolled a limited 4 Foulds J, O’Brien R. New tools for the diagnosis of tubercu-
losis: the perspective of developing countries. Int J Tuberc
number of HIV-coinfected patients and patients
Lung Dis 1998; 2:778 –783
with other comorbidities, decreasing its resemblance 5 Squire B. Tests to replace AFB microscopy in the diagnosis of
to most field-use conditions. The most pertinent smear positive pulmonary tuberculosis: meeting report.
group, persons with nontuberculous pulmonary dis- WHO Workshop on Tuberculosis Diagnostics, November,
ease, represented a minority of our study subjects, 1997. Available at: www.who.int/tdr/publications/publications/
though among individuals seeking care for respira- workshop.htm. Accessed December 11, 2002.
tory symptoms in primary care clinics they generally 6 Cavalcante SC, Pacheco AG, Lauria L, et al. Epidemiologia
da tuberculose no municı́pio do Rio de Janeiro: revisão dos
greatly outnumber TB patients. casos notificados de 1995 a 1997. Boletim de Pneumologia
Sanitária 1998; 6:81–92
7 Cole RA, Lu HM, Shi YZ, et al. Clinical evaluation of a rapid
immunochromatographic assay based on the 38-kd antigen of
Conclusion Mycobacterium tuberculosis on patients with pulmonary tu-
berculosis in China. Tuber Lung Dis 1996; 77:363–368
The principal findings of the current study are 8 Arloing S. Agglutination du bacille de la tuberculose vraie.
that serologic testing for TB with a simple mul- Comptes Rendues de l’Academie de Sciences 1898; 126:
tiantigen format is feasible in field settings with 1398 –1400
minimal technical requirements for the user. The 9 Daniel TM, Ellner JJ. Immunology of tuberculosis. In: Reich-
use of multiple antigens allowed detection of a man LB, Hershfield ES, eds. Tuberculosis: a comprehensive
international approach. New York, NY: Marcel Dekker, 1993;
significant number of additional patients over 75–101
those detected by the 38-kd band alone without a 10 Grange JM, Gibson J, Batty A. The specificity of the humoral
loss of specificity, but did not result in an overall immune response soluble mycobacterial antigens in tubercu-
sensitivity markedly greater than that reported in losis. Tubercle 1980; 61:153–156
previous studies. In HIV-uninfected patients, 11 Straus E, Wu N. Radioimmunoassay of the tuberculoprotein
derived from Mycobacterium tuberculosis. Proc Natl Acad
combined use of serology and sputum microscopy Sci U S A 1980; 77:4301– 4304
was as sensitive as culture, thus representing an 12 Straus E, Wu N, Quraishi MAH, et al. Clinical applications of
opportunity to greatly shorten the time to diagno- the radioimmunoassay of secretory tuberculoprotein. Proc
sis in a substantial subset of patients. As has been Natl Acad Sci U S A 1981; 78:3214 –3217
found in other serologic studies, test performance 13 Hewitt J, Coates ARM, Mitchison DA, et al. The use of
murine monoclonal antibodies without the purification of
in HIV co-infected patients in this study was poor, antigen in the serodiagnosis of tuberculosis. J Immunol
albeit superior to smear microscopy. Methods 1982; 55:205–211
Specificity was very high in healthy individuals and 14 Harboe M, Wiker HG. The 38-kd protein of Mycobacterium
was not compromised by tuberculin sensitivity or tuberculosis: a review. J Infect Dis 1992; 166:874 – 884
childhood BCG vaccination. The positive predictive 15 Anderson AB, Hansen EB. Structure and mapping of anti-
genic domains of protein b, a 38,000-molecular weight pro-
value in true use situations, however, was not calcu- tein of Mycobacterium tuberculosis. Infect Immun 1989;
lable due to the artificial composition of the study 57:2481–2488
population. Larger trials with more representative 16 Singh M, Andersen AM, McCarthy JEG, et al. The Mycobac-
populations are needed before the appropriate role terium tuberculosis 38-kd antigen: overproduction in Esche-
of TB serology can be ascribed. richia coli, purification and characterization. Gene 1992;
117:53– 60
ACKNOWLEDGMENT: We thank Dr. Draurio Barreira, Rio 17 McDonough JA, Sada ED, Sippola AA, et al. Microplate and
de Janeiro City DST/AIDS Program Coordinator; Dr. Guida dot immunoassays for the serodiagnosis of tuberculosis. J Lab
Vasconcelos, Rio de Janeiro City Tuberculosis Program Coordi- Clin Med 1992; 120:318 –322
www.chestjournal.org CHEST / 123 / 1 / JANUARY, 2003 111
Downloaded From: http://journal.publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21987/ on 04/20/2017
18 Lee B, Hefta SA, Brennan PJ. Characterization of the major 24 Bothamley GH, Rudd R, Festenstein F, et al. Clinical
membrane protein of virulent Mycobacterium tuberculosis. value of the measurement of Mycobacterium tuberculosis
Infect Immun 1992; 69:2006 –2074 specific antibody in pulmonary tuberculosis. Thorax 1992;
19 Bothamley GH, Schreuder GMT. Human leukocyte antigen, 47:270 –275
tuberculosis, and Mycobacterium tuberculosis-specific anti- 25 Roth A, Schaberg T, Mauch H. Molecular diagnosis of
body [letter]. J Infect Dis 1992; 165:598 tuberculosis: current clinical validity and future perspectives.
20 Bothamley GH. Serologic diagnosis of tuberculosis. Eur Eur Respir J 1997; 10:1877–1891
Respir J Suppl 1995; 20:676s– 688s 26 Zhou AT, Ma WL, Zhang PY, et al. Detection of pulmonary and
21 Lyashchenko K, Colangeli R, Houde M, et al. Heterogeneous extrapulmonary tuberculosis patients with the 38-kd antigen
antibody responses in tuberculosis. Infect Immun 1998; from Mycobacterium tuberculosis in a rapid membrane-based
66:3936 –3940 assay. Clin Diagn Lab Immunol 1996; 3:337–341
22 Hoeppner V, Mayers I. The antibody response to primary 27 Mathur ML, LoBue PA, Catanzaro A. Evaluation of a
tuberculous infection in man. Chest 1989; 95:171S serologic test for the diagnosis of tuberculosis. Int J Tuberc
23 Verbon A, Weverling GJ, Kuijper S, et al. Evaluation of Lung Dis 1999; 3:732–735
different tests for the serodiagnosis of tuberculosis and the 28 Freeman R, Magee J, Barratt A, et al. Rapid immunochromato-
use of likelihood ratios in serology. Am Rev Respir Dis 1993; graphic assay for diagnostics of tuberculosis: antibodies detected
148:378 –384 may not be specific. J Clin Microbiol 1999; 37:2111–2112
112 Clinical Investigations
Downloaded From: http://journal.publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21987/ on 04/20/2017
You might also like
- Infections in Cancer Chemotherapy: A Symposium Held at the Institute Jules Bordet, Brussels, BelgiumFrom EverandInfections in Cancer Chemotherapy: A Symposium Held at the Institute Jules Bordet, Brussels, BelgiumNo ratings yet
- Eclinicalmedicine: Research PaperDocument8 pagesEclinicalmedicine: Research PaperChacanNo ratings yet
- Pi Is 1201971213002142Document5 pagesPi Is 1201971213002142melisaberlianNo ratings yet
- Jurnal Respi - Comparison of IGRA AssayDocument5 pagesJurnal Respi - Comparison of IGRA AssaykaynabilNo ratings yet
- Gene Therapy Clinical Trials Worldwide To 2017 An UpdateDocument16 pagesGene Therapy Clinical Trials Worldwide To 2017 An UpdateLeonardo Sandi SalazarNo ratings yet
- PPT HiponatremiDocument9 pagesPPT HiponatremiArini NurlelaNo ratings yet
- NXJDBSKHDDocument4 pagesNXJDBSKHDMonika ZikraNo ratings yet
- Diagnostic Potential Of 16 Kda (Hspx, Α-Crystalline) Antigen For Serodiagnosis Of TuberculosisDocument7 pagesDiagnostic Potential Of 16 Kda (Hspx, Α-Crystalline) Antigen For Serodiagnosis Of TuberculosispouralNo ratings yet
- PIIS1201971218345090Document9 pagesPIIS1201971218345090favorendaNo ratings yet
- Detection of Typhoid CarriersDocument6 pagesDetection of Typhoid CarriersClarestaNo ratings yet
- American Journal of Infection Control: Major ArticleDocument5 pagesAmerican Journal of Infection Control: Major ArticleSianiiChavezMoránNo ratings yet
- Genexpert For Diagnosis of Tubercular Meningitis: Clinical BriefDocument3 pagesGenexpert For Diagnosis of Tubercular Meningitis: Clinical BriefYas MiineNo ratings yet
- 2 - Adult Meningitis in A Setting of High HIV and TB Prevalence - Findings From 4961 Suspected Cases 2010 (Modelo para o Trabalho)Document6 pages2 - Adult Meningitis in A Setting of High HIV and TB Prevalence - Findings From 4961 Suspected Cases 2010 (Modelo para o Trabalho)SERGIO LOBATO FRANÇANo ratings yet
- A. S. Bhatia, Satish Kumar and B. C. Harinath: Author For CorrespondenceDocument5 pagesA. S. Bhatia, Satish Kumar and B. C. Harinath: Author For CorrespondencePROFE EFRA LAGRANGENo ratings yet
- Extrapulmonary Tuberculosis Comparative Analysis of Diagnostic Modalities Used in A Tertiary Healthcare SettingDocument2 pagesExtrapulmonary Tuberculosis Comparative Analysis of Diagnostic Modalities Used in A Tertiary Healthcare SettingBIOMEDSCIDIRECT PUBLICATIONSNo ratings yet
- The Diagnostic Value of Neutrophil CD64 in Detection of Sepsis in ChildrenDocument5 pagesThe Diagnostic Value of Neutrophil CD64 in Detection of Sepsis in ChildrenrinaviviaudianaNo ratings yet
- Clostridium Difficile Testing in The Clinical Laboratory: by Use of Multiple Testing AlgorithmsDocument5 pagesClostridium Difficile Testing in The Clinical Laboratory: by Use of Multiple Testing AlgorithmswnafikaNo ratings yet
- Jurnal HibridisasiDocument12 pagesJurnal HibridisasiNurfanida Natasya mNo ratings yet
- Penelitian AbstraktifDocument9 pagesPenelitian AbstraktifYondi Piter PapulungNo ratings yet
- A Diagnostic Rule For Tuberculous Meningitis: Rashmi Kumar, S N Singh, Neera KohliDocument4 pagesA Diagnostic Rule For Tuberculous Meningitis: Rashmi Kumar, S N Singh, Neera KohliAri WirantariNo ratings yet
- Comparative Evaluation of Real-Time Screening PCR Assays For Giardia Duodenalis and of Assays Discriminating The Assemblages A and BDocument12 pagesComparative Evaluation of Real-Time Screening PCR Assays For Giardia Duodenalis and of Assays Discriminating The Assemblages A and BFlávia UchôaNo ratings yet
- Gutierrez 2016Document2 pagesGutierrez 2016MCINo ratings yet
- Genexpert - On Body Fluid SpecimensDocument4 pagesGenexpert - On Body Fluid Specimensramo G.No ratings yet
- Molecularapproachesand Biomarkersfordetectionof MycobacteriumtuberculosisDocument14 pagesMolecularapproachesand Biomarkersfordetectionof MycobacteriumtuberculosischiralicNo ratings yet
- Detection of Tuberculosis Using Digital Chest RadiographyDocument9 pagesDetection of Tuberculosis Using Digital Chest RadiographyRachnaNo ratings yet
- Jurnal Mikro-2Document8 pagesJurnal Mikro-2Mila KarmilaNo ratings yet
- Diagnosis and Testing in Bronchiolitis: A Systematic ReviewDocument8 pagesDiagnosis and Testing in Bronchiolitis: A Systematic ReviewKathy JunesNo ratings yet
- DownloadDocument8 pagesDownloadFiona ValenciaNo ratings yet
- Use of Indicators For Good Infection Control PracticeDocument2 pagesUse of Indicators For Good Infection Control PracticeZY YanNo ratings yet
- MastitisDocument5 pagesMastitisAnonymous Kv0sHqFNo ratings yet
- Clinchem 2017 273698 FullDocument10 pagesClinchem 2017 273698 FullMaisyaroh SaragihNo ratings yet
- Atorvastatin Improves Sputum Conversion and Chest X-Ray Severity ScoreDocument6 pagesAtorvastatin Improves Sputum Conversion and Chest X-Ray Severity Scorecharmainemargaret.parreno.medNo ratings yet
- 2 C PDFDocument14 pages2 C PDFAvisena AzisNo ratings yet
- Clinical Microbiology Newsletter: Genexpert Testing: Applications For Clinical Microbiology, Part IDocument5 pagesClinical Microbiology Newsletter: Genexpert Testing: Applications For Clinical Microbiology, Part ImagicianchemistNo ratings yet
- ASMNGS 2018 AbstractsDocument187 pagesASMNGS 2018 AbstractsturnersdNo ratings yet
- Microbial Diagnostic NTMDocument12 pagesMicrobial Diagnostic NTMdicky wahyudiNo ratings yet
- Artigo 02 Geneexpert TBDocument4 pagesArtigo 02 Geneexpert TBpriscila pimentel martinelliNo ratings yet
- Salmonella Enterica Serotype Typhi (S. Typhi) - Malaria andDocument6 pagesSalmonella Enterica Serotype Typhi (S. Typhi) - Malaria andTunde EgunjobiNo ratings yet
- PIIS2214109X23001353Document14 pagesPIIS2214109X23001353Joaquin OlivaresNo ratings yet
- Jurnal 2Document12 pagesJurnal 2zingioNo ratings yet
- Jurnal 2Document10 pagesJurnal 2GFORCE .ID.No ratings yet
- Vih Actualizacion en OportunistasDocument18 pagesVih Actualizacion en Oportunistassamuro625No ratings yet
- Faring It IsDocument8 pagesFaring It IsMuhammad sukronNo ratings yet
- Molecular Approaches To Diagnosing and Managing Infectious Diseases: Practicality and CostsDocument7 pagesMolecular Approaches To Diagnosing and Managing Infectious Diseases: Practicality and CostsIsrar NasirNo ratings yet
- Jurnal 2Document9 pagesJurnal 2terenadyaputriNo ratings yet
- 149 FullDocument10 pages149 Fullসোমনাথ মহাপাত্রNo ratings yet
- Sépsis em Pacientes Neurovascular Focus On Infection and Sepsis in Intensive Care PatientsDocument3 pagesSépsis em Pacientes Neurovascular Focus On Infection and Sepsis in Intensive Care PatientsEdson MarquesNo ratings yet
- Poster Abstracts - OFID 2019:6 (Suppl 2) - S297Document1 pagePoster Abstracts - OFID 2019:6 (Suppl 2) - S297ELIONo ratings yet
- (1 → 3) -Β - Glucan-Guided Antifungal Therapy In Adults With Sepsis: The Candisep Randomized Clinical TrialDocument11 pages(1 → 3) -Β - Glucan-Guided Antifungal Therapy In Adults With Sepsis: The Candisep Randomized Clinical TrialwiwiNo ratings yet
- Protective Effect of BCG Against Tuberculous Meningitis and Miliary Tuberculosis: A Meta-AnalysisDocument5 pagesProtective Effect of BCG Against Tuberculous Meningitis and Miliary Tuberculosis: A Meta-AnalysisLely KarolinaNo ratings yet
- Genotypic Characterization of Mycobacterium Tuberculosis Strains Resistant To Rifampicin Isoniazid and Second Line Antibiotics in ChadDocument14 pagesGenotypic Characterization of Mycobacterium Tuberculosis Strains Resistant To Rifampicin Isoniazid and Second Line Antibiotics in ChadAthenaeum Scientific PublishersNo ratings yet
- 2017risks of Infection and Mortality Among Patients Colonized With Klebsiella Pneumoniae Carbapenemase Producing K. Pneumoniae Validation of Scores and Proposal For ManagementDocument7 pages2017risks of Infection and Mortality Among Patients Colonized With Klebsiella Pneumoniae Carbapenemase Producing K. Pneumoniae Validation of Scores and Proposal For Managementpastorizaariana25No ratings yet
- ContentServer AspDocument2 pagesContentServer AspDarian EnmanuelNo ratings yet
- Perspectives On Advances in Tuberculosis Diagnostics, Drugs, and VaccinesDocument17 pagesPerspectives On Advances in Tuberculosis Diagnostics, Drugs, and Vaccinesyenny handayani sihiteNo ratings yet
- Early Yuri Cintia 17360053 Pembimbing: Dr. Aspri Sulanto, Sp.ADocument12 pagesEarly Yuri Cintia 17360053 Pembimbing: Dr. Aspri Sulanto, Sp.APaomo Zhixia EarlyNo ratings yet
- Sun 2012Document6 pagesSun 2012ntnquynhproNo ratings yet
- Validacion CPISDocument5 pagesValidacion CPISXavier AbrilNo ratings yet
- Film Array PDFDocument10 pagesFilm Array PDFW Antonio Rivera MartínezNo ratings yet
- Evaluation of Sensitivity and Specificity of ELISA Against Widal Test For Typhoid Diagnosis in Endemic Population of KathmanduDocument8 pagesEvaluation of Sensitivity and Specificity of ELISA Against Widal Test For Typhoid Diagnosis in Endemic Population of KathmanduRiriMutammimaRizqiyaniNo ratings yet
- GeneXpert For TB Diagnosis PDFDocument6 pagesGeneXpert For TB Diagnosis PDFMutiara BaruNo ratings yet
- Ayesha Umar Wahedi - CV 2016Document4 pagesAyesha Umar Wahedi - CV 2016TanveerNo ratings yet
- Ayesha S. Mahmud: Current PositionDocument5 pagesAyesha S. Mahmud: Current PositionTanveerNo ratings yet
- AyeshaDocument3 pagesAyeshaTanveerNo ratings yet
- 14145527801159Document9 pages14145527801159TanveerNo ratings yet
- Curriculum VitaeDocument4 pagesCurriculum VitaeTanveerNo ratings yet
- Ayesha KausarDocument3 pagesAyesha KausarTanveerNo ratings yet
- Quran Ki MarifatDocument72 pagesQuran Ki MarifatTanveerNo ratings yet
- Curriculum Vitae: Dr. Sartaj Alam SyedDocument8 pagesCurriculum Vitae: Dr. Sartaj Alam SyedTanveerNo ratings yet
- DR Shaukat (Plant Pathology)Document5 pagesDR Shaukat (Plant Pathology)Tanveer100% (1)
- CV - Gaurav BahlDocument17 pagesCV - Gaurav BahlTanveerNo ratings yet
- Europass Curriculum Vitae: Personal Information Popescu Simona-AlinaDocument11 pagesEuropass Curriculum Vitae: Personal Information Popescu Simona-AlinaTanveerNo ratings yet
- Shaukat Ali: Curriculum VitaeDocument8 pagesShaukat Ali: Curriculum VitaeTanveerNo ratings yet
- Ali, Shaukat CV - Pubs Fy16 - 0Document3 pagesAli, Shaukat CV - Pubs Fy16 - 0TanveerNo ratings yet
- Curriculum Vitae: Dr. Tayyab SubhaniDocument13 pagesCurriculum Vitae: Dr. Tayyab SubhaniTanveerNo ratings yet
- Bio Tech Shankar Ayaz AhmadDocument14 pagesBio Tech Shankar Ayaz AhmadTanveerNo ratings yet
- Resume Muhammad Asif Sadiq Deputy Registrar GC University LahoreDocument1 pageResume Muhammad Asif Sadiq Deputy Registrar GC University LahoreTanveer100% (1)
- 1359443692128Document10 pages1359443692128TanveerNo ratings yet
- Minutes of Pre-Bid Meeting & Procurement of Consultancy Services To Conduct Feasibility Study For Establishing Monorail System Along Lahore Canal Road 17012014 0 0 2Document92 pagesMinutes of Pre-Bid Meeting & Procurement of Consultancy Services To Conduct Feasibility Study For Establishing Monorail System Along Lahore Canal Road 17012014 0 0 2TanveerNo ratings yet
- CV 22 09 2018Document37 pagesCV 22 09 2018TanveerNo ratings yet
- DR Shah (New CV)Document7 pagesDR Shah (New CV)TanveerNo ratings yet
- Wajid CVDocument13 pagesWajid CVTanveerNo ratings yet
- Curriculum Vitae Muhammad Wajid Raza: 1.contact InformationDocument4 pagesCurriculum Vitae Muhammad Wajid Raza: 1.contact InformationTanveer100% (1)
- Hussain I - CV 2016 - LumsDocument8 pagesHussain I - CV 2016 - LumsTanveerNo ratings yet
- Interneelist2013 PDFDocument40 pagesInterneelist2013 PDFTanveerNo ratings yet
- Salaheddin R. Malkawi: Salahm@just - Edu.joDocument8 pagesSalaheddin R. Malkawi: Salahm@just - Edu.joTanveerNo ratings yet
- Curriculum Vitae: Area of InterestDocument18 pagesCurriculum Vitae: Area of InterestTanveerNo ratings yet
- DR Munir AhmadDocument12 pagesDR Munir AhmadTanveerNo ratings yet
- Muhammad Yasir Yaqoob: ContactDocument2 pagesMuhammad Yasir Yaqoob: ContactTanveerNo ratings yet
- Rana Rubnawaz CV - 2014Document2 pagesRana Rubnawaz CV - 2014TanveerNo ratings yet
- Curriculum Vitae: Dr. Sohail AhmadDocument7 pagesCurriculum Vitae: Dr. Sohail AhmadTanveerNo ratings yet
- AsdasdDocument15 pagesAsdasdJohn Paulo RamosNo ratings yet
- Drug Study CompilationDocument9 pagesDrug Study CompilationRene John FranciscoNo ratings yet
- Lencastre Et Al-2019-Journal of The European Academy of Dermatology and VenereologyDocument2 pagesLencastre Et Al-2019-Journal of The European Academy of Dermatology and VenereologyMaria MogosNo ratings yet
- Alice in Wonderland Syndrome - EditedDocument9 pagesAlice in Wonderland Syndrome - Editedcharles wambuiNo ratings yet
- Rare Diseases 2013 PDFDocument56 pagesRare Diseases 2013 PDFantikenomaNo ratings yet
- Typhoid Enteric Fever From UiA Volume 32 Final WebDocument4 pagesTyphoid Enteric Fever From UiA Volume 32 Final WebJayesh KumarNo ratings yet
- McCance Patho Review QsDocument17 pagesMcCance Patho Review Qsiamdara100% (1)
- Syndrome of Inappropriate Vasopressin Sexretion (Siadh)Document22 pagesSyndrome of Inappropriate Vasopressin Sexretion (Siadh)Moni RethNo ratings yet
- SDCEP Drug Prescribing Ed 3 Update June 2021Document4 pagesSDCEP Drug Prescribing Ed 3 Update June 2021ABNo ratings yet
- Non-Invasive Positive Pressure Ventilation (Nippv)Document4 pagesNon-Invasive Positive Pressure Ventilation (Nippv)docsandyy78No ratings yet
- Non-Communicable Diseases and Physical InactivityDocument37 pagesNon-Communicable Diseases and Physical InactivityMiles MontinolaNo ratings yet
- Head Injury Lect 5Document27 pagesHead Injury Lect 5amber tariq100% (1)
- Apogeotropic Variant of Posterior Canal Benign Paroxysmal Positional VertigoDocument7 pagesApogeotropic Variant of Posterior Canal Benign Paroxysmal Positional VertigojorgepierreNo ratings yet
- Drug StudyDocument10 pagesDrug Studykierjohn237343No ratings yet
- Rectal Prolapse: DR Anas Ahmad PGR - Surgical Unit 2 Shalamar Hospital-LahoreDocument37 pagesRectal Prolapse: DR Anas Ahmad PGR - Surgical Unit 2 Shalamar Hospital-LahoreReshu ThakuriNo ratings yet
- ConjungtivitaDocument85 pagesConjungtivitaFatrisNo ratings yet
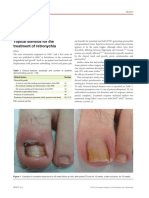
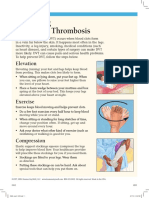
- Preventing Deep Vein Thrombosis: ElevationDocument2 pagesPreventing Deep Vein Thrombosis: ElevationTeuku Ilham AkbarNo ratings yet
- Tabel JurnalDocument11 pagesTabel JurnalEddy KurniawanNo ratings yet
- PSYCH-POST-TESTDocument27 pagesPSYCH-POST-TESTAdine Jeminah LimonNo ratings yet
- HyperthyroidDocument12 pagesHyperthyroidChristine Joy PepitoNo ratings yet
- Placenta PreviaDocument3 pagesPlacenta PreviaReyniel Pablo ElumbaNo ratings yet
- First Aid PPT - DR GhaziDocument22 pagesFirst Aid PPT - DR GhaziMands_KLP100% (1)
- Ankle SprainDocument3 pagesAnkle SpraindrakbarbabNo ratings yet
- The Five Commandments of Infectious Disease ControlDocument2 pagesThe Five Commandments of Infectious Disease ControlCarol ReedNo ratings yet
- Lumbar Myofascial Pain SyndromeDocument8 pagesLumbar Myofascial Pain SyndromeYani Desi YantiNo ratings yet
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDocument112 pagesOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreYoAmoNYC100% (2)
- Glaukoma CSDocument13 pagesGlaukoma CSKevinVun100% (4)
- Hemolytic AnemiaDocument99 pagesHemolytic AnemiaSagar Chandrakant Mhetre100% (3)
- Epigastric Pain and Management of FeverDocument41 pagesEpigastric Pain and Management of FeverAkhr RiNo ratings yet
- Pityriasis AmiantaceaDocument4 pagesPityriasis AmiantaceaWidia WidiaaNo ratings yet