Professional Documents
Culture Documents
Part 1 51-11-01: Corrosion Prevention Manual
Uploaded by
clebersjcOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Part 1 51-11-01: Corrosion Prevention Manual
Uploaded by
clebersjcCopyright:
Available Formats
CORROSION
PREVENTION MANUAL
TASK 51-11-01-910-001-A
EFFECTIVITY: ALL
TYPES AND CAUSES OF CORROSION
1. General
A. Corrosion is a phenomenon that occurs in metals as a result of chemical or electrochemical action.
With time and usage, metals go back to a low-energy state and change into metal compounds such as
oxides, hydroxides or sulfates.
B. Because different materials have different characteristics and properties, they can be under the effects
of corrosion. Other factors that cause corrosion are environmental conditions. To prevent or decrease
the effects of corrosion, research on new materials never stops. It is necessary to improve the protec-
tion of metal parts.
C. To understand the procedures for the prevention and control of metal corrosion, it is necessary to
know:
• The appearance and types of corrosion to which the different types of metals are subject.
• The corrosive agents that attack the metal surfaces.
• The areas of the aircraft that are usually under corrosive attack.
• The classification of corrosion levels.
• The different types of repair for each type of material.
D. These are the best procedures for protection against corrosion:
• The use of corrosion-resistant materials.
• Good protection for the materials in the design phase and the service life of the components.
• Cleaning and regular maintenance of the aircraft.
• Immediate identification of corrosion.
• Immediate repair of corroded parts, components and areas.
Even when you take these precautions, corrosion can occur as a result of special climatic conditions or
because of the influence of substances such as salts and contaminants.
2. Causes of Corrosion
A. Corrosion starts when these conditions occur:
• There is a metal subject to corrosion (anode).
• There is a dissimilar conductive material (cathode).
• There is a continuous conductive liquid path (electrolyte).
• There is an electric contact between the anode and the cathode.
B. These are other causes of corrosion:
• The incorrect choice of materials, manufacturing process, and assembly procedures.
Embraer 195 - CPM 4132 910-001-A
Part 1 51-11-01 Page 1 of 6
Nov 25/10
Copyright © 2010 by Embraer - Empresa Brasileira de Aeronáutica S.A. All rights reserved.
CORROSION
PREVENTION MANUAL
• The usual operation and maintenance of the aircraft (removal of the protective finish).
• Environmental conditions (seacoast humidity, airborne pollutants).
• Finish deterioration (age and erosion).
• Aircraft operation (animal transportation and seafood shipment).
• Accidental contamination (chemical spills and galley spillage).
3. Corrosive Agents
A. Many fluids used in aircraft maintenance and operation are corrosive. The climate and atmospheric
conditions are also corrosive. The most common corrosive agents are:
(1) Acids.
(a) Sulfuric acid (battery acid) and organic acid (human and animal waste) can cause corrosion
in many alloys.
(2) Alkaline Solutions.
(a) Alkaline solutions can cause corrosion in aluminum alloys. The most common alkaline solu-
tions are washing soda and potash.
(3) Salts.
(a) Salt solutions can attack aluminum alloys and steels. The salt solutions are corrosive
agents because they are good electrolytes.
(4) Corrosive Agents in the Atmosphere.
(a) The two primary corrosive agents in the atmosphere are moisture and oxygen. The environ-
mental conditions, such as those in industrial and marine regions, can increase atmospher-
ic corrosion.
1 Industrial Environment
a The most common agents are oxidized sulfur and nitrogen compounds. When
one of these agents mix with moisture, it becomes a corrosive agent and at-
tacks the aircraft structure.
2 Marine Environment
a Airborne salts in a marine environment is one of the most corrosive agents. This
is because the salt solutions are electrolytes and can cause corrosion in the air-
craft structure.
4. Types of Corrosion
The most common types of corrosion are:
A. Galvanic Corrosion.
Embraer 195 - CPM 4132 910-001-A
Part 1 51-11-01 Page 2 of 6
Nov 25/10
Copyright © 2010 by Embraer - Empresa Brasileira de Aeronáutica S.A. All rights reserved.
CORROSION
PREVENTION MANUAL
(1) Maintenance personnel can recognize this type of corrosion because it leaves a deposit of white
or gray powder in metal joints. Galvanic corrosion occurs when two dissimilar metals make con-
tact or when there is an electrolyte between them. For galvanic corrosion to occur, there must be
an anode (a material more subject to corrosion) and a cathode (a material less subject to corro-
sion). A protective finish can decrease the effects and the rate of corrosion. But when a mainte-
nance procedure causes the removal of the protective finish, the galvanic corrosion process can
start again.
B. Crevice Corrosion.
(1) This type of corrosion is severe and occurs locally between interfaying surfaces. Crevice corro-
sion is the result of electrochemical action. This phenomenon occurs in the same way as galvanic
corrosion, where two dissimilar metals act as an anode and a cathode. Water, which has oxygen,
gets into the crevice and becomes an electrolyte. It is difficult to find crevice corrosion in the be-
ginning of the process because it occurs in hidden areas.
(2) Crevice corrosion becomes more severe when it contains salts (usually in seacoast environ-
ments, galleys, lavatories, cargo spills, and human waste).
(3) The use of water-displacing compounds can help prevent this type of corrosion or decrease its
effects. But you must keep the layer of compound in good condition to prevent the start of the
corrosion process.
C. Pitting Corrosion.
(1) Maintenance personnel can recognize this type of corrosion because of the pits (holes) in the
metal surfaces. Pitting corrosion starts when the protective finish is not of the correct type or
when it is damaged. Pitting corrosion can start, go into a given depth, and then stop. This type of
corrosion can cause many small local pits in different areas, with almost no loss of material. So,
the remainder of the metal surface stays in almost good condition. But these pits can spread and
go fully through the metal.
(2) Pitting corrosion usually occurs when erosion or other forms of corrosion starts. Pits (holes) in a
crevice increases at a higher rate than those on a visible surface. It can cause fatigue cracks to
start and spread. The correct protective treatment can prevent or stop this type of corrosion.
D. Exfoliation Corrosion.
(1) This corrosion is of the intergranular type and causes flaking and loss of metal thickness. Exfolia-
tion corrosion occurs because of the swelling and flaking at the grain ends during the manufactur-
ing process. When the machining and chemical milling operations show the end of the grain, ex-
foliation corrosion can occur. To prevent exfoliation corrosion, the metal must receive sufficient
protection. Exfoliation corrosion can cause bulging on the faying surface of skin panels. This will
cause fastener head deformation, pull-through, or failure.
E. Microbiological Corrosion.
(1) Microorganisms can cause this type of corrosion. Maintenance personnel can recognize micro-
biological corrosion because it contains fungi. It looks slimy with a color range from brown to
black. It takes the shape of long threads and forms mats or scattered globules. Contaminated fuel
can cause microbiological corrosion. Microorganisms release metabolic products that attack the
tank finish and corrode aluminum alloys.
F. Fretting Corrosion.
Embraer 195 - CPM 4132 910-001-A
Part 1 51-11-01 Page 3 of 6
Nov 25/10
Copyright © 2010 by Embraer - Empresa Brasileira de Aeronáutica S.A. All rights reserved.
CORROSION
PREVENTION MANUAL
(1) This type of corrosion occurs because of the removal of the protective film on the metal surface.
The bare metal is subject to wear and corrosion because of the movement of the metal surfaces
against each other. This forms layers of dark oxides. Debris and corrosive agents can increase
the corrosion process. Because of this, seizure and cracks can occur at the joints. The cracks at
the joints will spread as a result of cyclic loading.
G. Intergranular Corrosion.
(1) Intergranular corrosion is an attack on the grain boundaries of a metal. Microphotographs show
that aluminum alloys consist of very small grains held together by chemical bonds.
In the heat-treating process, the temperature of the metal increases so much that the alloying el-
ements go into solution with each other. When the temperature of the metal is uniform, the metal
is removed from the furnace and immediately quenched in water. This solidifies the alloying ele-
ments into small grains.
If there is a delay in the quenching procedure, even for a few seconds, the grains will grow. The
grains will grow to such a size that the areas of dissimilar metals will have efficient anodes and
cathodes to start the corrosion process.
If the corrosion gets to the boundary between the large grains through a pit (hole), the corrosion
process can continue in the metal. The electrolyte comes from the corrosion deposits on the met-
al surface and along the grain boundaries while the intergranular corrosion process continues.
(2) Spot welds or seam welds can also make the grains larger and cause intergranular corrosion.
Under the metal surface, there can be blisters removed from the point of initial penetration. The
metal surface over these blisters is very thin. If you lift the metal surface with the point of a knife,
you will see a cavity filled with the salts of corrosion.
(3) Because intergranular corrosion occurs in the metal (not on the surface), it is difficult to find it
without the use of ultrasonic or X-ray equipment. If a part shows signs of intergranular corrosion,
the only correct procedure is to replace the damaged part.
H. Stress Corrosion.
(1) Maintenance personnel can recognize this type of corrosion because the metal shows cracks that
spread quickly. It is a type of intergranular corrosion that starts as a result of residual stresses
from machining or chemical milling operations. The bare metal surface is subject to corrosive at-
tack.
(2) Stress corrosion usually occurs in areas where there are open grains or pits. It can cause the de-
struction of the structural components. The careful choice of materials, correct design, good as-
sembly techniques, and surface protection can prevent this type of corrosion.
5. Areas Where Corrosion Can Occur (Critical Areas)
A. The operator must give special attention to the conditions of the areas where corrosion usually occurs.
These conditions are:
• Cleanliness;
• Frequency of inspection;
• Application of corrosion-inhibiting compounds;
• Treatment of the area where corrosion occurred.
Embraer 195 - CPM 4132 910-001-A
Part 1 51-11-01 Page 4 of 6
Nov 25/10
Copyright © 2010 by Embraer - Empresa Brasileira de Aeronáutica S.A. All rights reserved.
CORROSION
PREVENTION MANUAL
B. The areas where corrosion usually occurs are:
(1) Bilge Area.
(a) Dirt and contaminants, which can become corrosive agents, usually collect in the bilge
area. They are:
• Hydraulic fluid
• Water
• Chips
• Lost fasteners
(2) Lavatory Area - Including Floor and Underfloor Area.
(a) All areas near the lavatory are subject to spills of fluids (water, cleaning products, etc.), dirt,
and leaks in the lavatory pump line.
(3) Galley Area - Including Floor, Underfloor Area, Drawers, Drawer Track, and Seat Tracks near this
area.
(a) All areas near the galley are subject to spills of fluids (water, cleaning products, beverages,
etc.) which, together with leftovers and dirt, collect on the drawer tracks, in the drawers, and
on the tracks of the passenger seats.
(4) Nose and Main Landing Gear Wheelwells and Doors.
(a) These are some of the areas where corrosion usually occurs because of the direct action of
water, moisture, debris, salts, sand, gravel, and pebbles.
(5) Passenger and Cargo Doors and Surrounding Structure.
(a) During boarding, deplaning, and loading and unloading operations, moisture and rainwater
can get into these areas. Marine and industrial environments can also cause corrosion in
these areas.
(6) Battery Compartment.
(a) The operator must give special attention to this area because it is difficult to prevent battery
acid spillage during servicing. A leak in a damaged battery is also a cause of corrosion.
Spilled battery acid can collect in cavities of the aircraft structure and spread to areas that
do not have good protection.
(7) Skin Areas, Aft Fuselage and Horizontal Stabilizers.
(a) In these areas, corrosion usually occurs at the edges of the skin panels and around the fas-
tener heads. The operator must give special attention to the areas where the maintenance
personnel replaced the fasteners. This is because the fastener holes can become a source
of corrosion. The most common type of corrosion that occurs on skin panel surfaces is the
filiform corrosion.
(8) Fuel Tanks.
Embraer 195 - CPM 4132 910-001-A
Part 1 51-11-01 Page 5 of 6
Nov 25/10
Copyright © 2010 by Embraer - Empresa Brasileira de Aeronáutica S.A. All rights reserved.
CORROSION
PREVENTION MANUAL
(a) Corrosion in the fuel tank can occur because microorganisms get into it during the filling op-
eration. Water dissolved or suspended in the fuel is also a source of corrosion. Contamina-
tion of the fuel because of microbial growth usually occurs in aircraft that operate in hot,
damp climate.
Embraer 195 - CPM 4132 910-001-A
Part 1 51-11-01 Page 6 of 6
Nov 25/10
Copyright © 2010 by Embraer - Empresa Brasileira de Aeronáutica S.A. All rights reserved.
You might also like
- Chapter 5 - CORROSION AND NON-FERROUS METALDocument60 pagesChapter 5 - CORROSION AND NON-FERROUS METALتاج نيسها33% (3)
- NRL Painting ManualDocument86 pagesNRL Painting ManualopalakalakaNo ratings yet
- Chemistry - Encyclopedia of Electrochemistry, Volume 04 Corrosion and Oxide Films) - (Martin Stratmann, Gerald S Frankel) Wiley 2007 PDFDocument696 pagesChemistry - Encyclopedia of Electrochemistry, Volume 04 Corrosion and Oxide Films) - (Martin Stratmann, Gerald S Frankel) Wiley 2007 PDFlovaldes62No ratings yet
- Oil and Gas Corrosion Prevention: From Surface Facilities to RefineriesFrom EverandOil and Gas Corrosion Prevention: From Surface Facilities to RefineriesRating: 5 out of 5 stars5/5 (6)
- Corrosion: Corrosion ControlFrom EverandCorrosion: Corrosion ControlL L ShreirRating: 5 out of 5 stars5/5 (1)
- Corrosion and its Consequences for Reinforced Concrete StructuresFrom EverandCorrosion and its Consequences for Reinforced Concrete StructuresNo ratings yet
- MPP3914 - 36 11 01 07 1Document8 pagesMPP3914 - 36 11 01 07 1clebersjcNo ratings yet
- Aircraft Maintenance Manual: Task Number Description EffectivityDocument10 pagesAircraft Maintenance Manual: Task Number Description EffectivityclebersjcNo ratings yet
- Configuration, Maintenance and Procedures: CMP-2925 REV. 0 Page 1C-Part 1CDocument4 pagesConfiguration, Maintenance and Procedures: CMP-2925 REV. 0 Page 1C-Part 1CclebersjcNo ratings yet
- MRB1928 Appendix-A PDFDocument82 pagesMRB1928 Appendix-A PDFclebersjcNo ratings yet
- Chapter 1 - IntroductionDocument20 pagesChapter 1 - IntroductionEDU Academic Programs CoordinatorNo ratings yet
- Presentation On CorrosionDocument7 pagesPresentation On CorrosionBemgbaNo ratings yet
- MODEL R172 SERIES (1977 - 1981) : Corrosion 1. GeneralDocument12 pagesMODEL R172 SERIES (1977 - 1981) : Corrosion 1. GeneralAntonio BedoyaNo ratings yet
- CORROSIONDocument13 pagesCORROSIONJuan TapiaNo ratings yet
- Chapter 2 Foundamental Theory of DamagesDocument19 pagesChapter 2 Foundamental Theory of DamagesabelNo ratings yet
- Unit 2 CorrosionDocument37 pagesUnit 2 CorrosionRohan PolNo ratings yet
- Corrosion and ControlDocument33 pagesCorrosion and ControlVishwasen Khot100% (1)
- AC43-ch6 - 1 CORROSION PDFDocument44 pagesAC43-ch6 - 1 CORROSION PDFFREDDYNo ratings yet
- Chapter 6. Corrosion, Inspection & Protection: 9/27/01 AC 43.13-1B CHG 1Document4 pagesChapter 6. Corrosion, Inspection & Protection: 9/27/01 AC 43.13-1B CHG 1blackhawkNo ratings yet
- ME1112 Engineers Guide To Corrosion Causes Protection and ControlDocument162 pagesME1112 Engineers Guide To Corrosion Causes Protection and ControlFarid TataNo ratings yet
- Classwork MSE542-442105835Document39 pagesClasswork MSE542-442105835Abdulaziz H. Al-MutairiNo ratings yet
- Corrosion Pre FinalsDocument11 pagesCorrosion Pre FinalsbnolascoNo ratings yet
- ChemistryDocument25 pagesChemistryHarish KumarNo ratings yet
- Afsa Corrosion Pocket GuideDocument36 pagesAfsa Corrosion Pocket GuideNuno PachecoNo ratings yet
- Aircraft CorrosionsDocument33 pagesAircraft Corrosionsheruizhe06No ratings yet
- Effect of Anodization On The Corrosion Behavior of Aluminium Alloy in HCL Acid and NaohDocument5 pagesEffect of Anodization On The Corrosion Behavior of Aluminium Alloy in HCL Acid and NaohGabriel DevaNo ratings yet
- Engineering Corrosion OH-7: University of Hafr Al BatinDocument27 pagesEngineering Corrosion OH-7: University of Hafr Al BatinHussain Al-DawoodNo ratings yet
- Corrosion BasicDocument500 pagesCorrosion BasicKing Sabi100% (1)
- Spray Painting Unit 1.2Document17 pagesSpray Painting Unit 1.2arun3kumar00_7691821No ratings yet
- CorrosionDocument29 pagesCorrosionAbdul-aziz RajabNo ratings yet
- Chapter 2 Foundamental Theories of DamagesDocument19 pagesChapter 2 Foundamental Theories of DamagesOusman ToficNo ratings yet
- Corrosion and Corrosion Control BookletDocument94 pagesCorrosion and Corrosion Control Bookletdss1234No ratings yet
- 01 Factors Affecting Corrosion - Types of CorrosionDocument33 pages01 Factors Affecting Corrosion - Types of CorrosionNino AngobNo ratings yet
- CORROSION IN MARINE ENVIRONMENT The Type PDFDocument5 pagesCORROSION IN MARINE ENVIRONMENT The Type PDFHotnCrispy CrispyNo ratings yet
- Control of Corrosion On Underwater PilesDocument14 pagesControl of Corrosion On Underwater Pilesವಿನಯ್ ಎಮ್. ಆರ್100% (1)
- Metallurgical Factors Affecting CorrosionDocument21 pagesMetallurgical Factors Affecting CorrosionCharlie Chong100% (6)
- Corrosion Engineering: Koya University Faculty of Engineering Petroleum Engineering Department 3 Stage 2016/2017Document13 pagesCorrosion Engineering: Koya University Faculty of Engineering Petroleum Engineering Department 3 Stage 2016/2017Ali AhmadNo ratings yet
- UNIT-I CorrDocument42 pagesUNIT-I CorrArthi SelvaNo ratings yet
- CorrosionDocument20 pagesCorrosionndesigngmailNo ratings yet
- Lec. 1 Corrosion - IntroductionDocument8 pagesLec. 1 Corrosion - IntroductionOmer IkhlasNo ratings yet
- Amtg - Handbook 325 353Document29 pagesAmtg - Handbook 325 353LA ONDA TROPICAL TV MEXICONo ratings yet
- Aircraft Corrosion and ControlDocument39 pagesAircraft Corrosion and ControlShaira LouiseNo ratings yet
- Unit 2 Corrosion - 2019Document31 pagesUnit 2 Corrosion - 2019Abhay hNo ratings yet
- Corrosion in The Oil Industry 1683626672Document51 pagesCorrosion in The Oil Industry 1683626672Rozil AnwarNo ratings yet
- Seminar ReportDocument8 pagesSeminar ReportLaxman HosamaniNo ratings yet
- CCUWP ArmashDocument25 pagesCCUWP ArmashArmash MominNo ratings yet
- Biomaterials Journal: Corrosive Failure of Metals and Alloys in Dentistry: A ReviewDocument6 pagesBiomaterials Journal: Corrosive Failure of Metals and Alloys in Dentistry: A ReviewIstiak MahmoodNo ratings yet
- Introduction To Corrosion and Its PreventionDocument29 pagesIntroduction To Corrosion and Its PreventionEsaKhanNo ratings yet
- Corrosion Lab ReportDocument21 pagesCorrosion Lab ReportJeremiah MolaletsiNo ratings yet
- Corrosion New Jul20Document66 pagesCorrosion New Jul20hafiz aimanNo ratings yet
- What Is CorrosionDocument4 pagesWhat Is CorrosionOsransyah Os100% (1)
- Week 1b IntroductionDocument44 pagesWeek 1b IntroductionAraasu EgambaramNo ratings yet
- Trends in Corrosion Management - FinalDocument11 pagesTrends in Corrosion Management - FinalLuís PiresNo ratings yet
- Corrosion Protection of SteelDocument9 pagesCorrosion Protection of SteelChristian D. OrbeNo ratings yet
- Beginners Guide To CorrosionDocument10 pagesBeginners Guide To Corrosionshamu081No ratings yet
- 201 A DocumentDocument35 pages201 A DocumentGRANRICKYNo ratings yet
- Corrosion of Stainless SteelDocument10 pagesCorrosion of Stainless SteelRizky Ilham DescarianNo ratings yet
- CCUWP ArmashDocument26 pagesCCUWP ArmashArmash MominNo ratings yet
- Corrosion in AviationDocument8 pagesCorrosion in AviationRiaz LourencoNo ratings yet
- Unit 3. Corrosion 2022Document31 pagesUnit 3. Corrosion 2022Rohit AgrawalNo ratings yet
- Akpanyung 2019 J. Phys. Conf. Ser. 1378 022088Document16 pagesAkpanyung 2019 J. Phys. Conf. Ser. 1378 022088Maysam MohamNo ratings yet
- Corrosion Issues and Prevention in Oil IndustryDocument33 pagesCorrosion Issues and Prevention in Oil IndustryPreet Singh100% (1)
- Cathodic Protection: Industrial Solutions for Protecting Against CorrosionFrom EverandCathodic Protection: Industrial Solutions for Protecting Against CorrosionNo ratings yet
- MPP3914 - 36 11 03 04 1Document20 pagesMPP3914 - 36 11 03 04 1clebersjcNo ratings yet
- Mrb1928 050 Othersect 4Document10 pagesMrb1928 050 Othersect 4clebersjcNo ratings yet
- Aircraft Maintenance Manual: Task Number Description EffectivityDocument6 pagesAircraft Maintenance Manual: Task Number Description EffectivityclebersjcNo ratings yet
- Part 1 51-00-01: Corrosion Prevention ManualDocument4 pagesPart 1 51-00-01: Corrosion Prevention ManualclebersjcNo ratings yet
- Aircraft Maintenance Manual: Task Number Description EffectivityDocument28 pagesAircraft Maintenance Manual: Task Number Description EffectivityclebersjcNo ratings yet
- MRB1928 040-ZonalDocument34 pagesMRB1928 040-ZonalclebersjcNo ratings yet
- MPP3914 - 36 11 00 05 1Document24 pagesMPP3914 - 36 11 00 05 1clebersjcNo ratings yet
- Aircraft Maintenance Manual: Task Number Description EffectivityDocument16 pagesAircraft Maintenance Manual: Task Number Description EffectivityclebersjcNo ratings yet
- MPP3914 - 36 11 01 05 1Document20 pagesMPP3914 - 36 11 01 05 1clebersjcNo ratings yet
- MPP3914 - 36 11 01 06 1Document4 pagesMPP3914 - 36 11 01 06 1clebersjcNo ratings yet
- CMP2925 010-Part1 PDFDocument2 pagesCMP2925 010-Part1 PDFclebersjcNo ratings yet
- MPP3914 - 36 11 01 04 1Document28 pagesMPP3914 - 36 11 01 04 1clebersjcNo ratings yet
- Aircraft Maintenance Manual: Task Number Description EffectivityDocument4 pagesAircraft Maintenance Manual: Task Number Description EffectivityclebersjcNo ratings yet
- MPP3914 - 36 10 01 07 1Document8 pagesMPP3914 - 36 10 01 07 1clebersjcNo ratings yet
- Mrb1928 Appendix ADocument8 pagesMrb1928 Appendix AclebersjcNo ratings yet
- Aircraft Maintenance Manual: Task Number Description EffectivityDocument6 pagesAircraft Maintenance Manual: Task Number Description EffectivityclebersjcNo ratings yet
- MPP3914 - 36 00 00 05 1Document16 pagesMPP3914 - 36 00 00 05 1clebersjcNo ratings yet
- Configuration, Maintenance and Procedures: Part 1 B - Apu ItemsDocument2 pagesConfiguration, Maintenance and Procedures: Part 1 B - Apu ItemsclebersjcNo ratings yet
- Configuration, Maintenance and Procedures: A - Engine ItemsDocument2 pagesConfiguration, Maintenance and Procedures: A - Engine ItemsclebersjcNo ratings yet
- Aircraft Maintenance Manual: Task Number Description EffectivityDocument22 pagesAircraft Maintenance Manual: Task Number Description EffectivityclebersjcNo ratings yet
- Aircraft StationsDocument16 pagesAircraft StationsclebersjcNo ratings yet
- Aircraft StationsDocument16 pagesAircraft StationsclebersjcNo ratings yet
- Aircraft Structural SectionDocument2 pagesAircraft Structural SectionclebersjcNo ratings yet
- Aircraft General DimensionsDocument4 pagesAircraft General DimensionsclebersjcNo ratings yet
- Aircraft General DimensionsDocument4 pagesAircraft General DimensionsclebersjcNo ratings yet
- The Explanation of The Fundamentals of Islamic BeliefDocument95 pagesThe Explanation of The Fundamentals of Islamic BeliefbooksofthesalafNo ratings yet
- AC350 Specs UsDocument18 pagesAC350 Specs Uskloic1980100% (1)
- Offsetting Macro-Shrinkage in Ductile IronDocument13 pagesOffsetting Macro-Shrinkage in Ductile IronmetkarthikNo ratings yet
- Zetor Crystal 150 170 Tractor Operator Manual PDFDocument234 pagesZetor Crystal 150 170 Tractor Operator Manual PDFAntonNo ratings yet
- 19 Work Energy TNDocument2 pages19 Work Energy TNAna DorueloNo ratings yet
- Calabano Clinical Bacteriology Exercise 1Document5 pagesCalabano Clinical Bacteriology Exercise 1MarkJasperCalabanoNo ratings yet
- Column c4 From 3rd FloorDocument1 pageColumn c4 From 3rd Floor1man1bookNo ratings yet
- KZPOWER Perkins Stamford Genset Range CatalogueDocument2 pagesKZPOWER Perkins Stamford Genset Range CatalogueWiratama TambunanNo ratings yet
- Latihan Soal BlankDocument8 pagesLatihan Soal BlankDanbooNo ratings yet
- Case-Study - Decision Making Under UncertaintyDocument21 pagesCase-Study - Decision Making Under UncertaintyMari GhviniashviliNo ratings yet
- Phonetics ReportDocument53 pagesPhonetics ReportR-jhay Mepusa AceNo ratings yet
- Wjec Biology SpectificaionDocument93 pagesWjec Biology SpectificaionLucy EvrettNo ratings yet
- ONGC Buyout GOI's Entire 51.11% Stake in HPCLDocument4 pagesONGC Buyout GOI's Entire 51.11% Stake in HPCLArpan AroraNo ratings yet
- Microsoft Word - IRN Fab Transfer PCN NoticeDocument22 pagesMicrosoft Word - IRN Fab Transfer PCN NoticeJadilson PradoNo ratings yet
- Estimation of Fire Loads For An Educational Building - A Case StudyDocument4 pagesEstimation of Fire Loads For An Educational Building - A Case StudyEditor IJSETNo ratings yet
- NCERT Solutions For Class 12 Maths Chapter 10 Vector AlgebraDocument51 pagesNCERT Solutions For Class 12 Maths Chapter 10 Vector AlgebraKavin .J.S (KingK)No ratings yet
- Smart Locker - A Sustainable Urban Last-Mile Delivery Solution: Benefits and Challenges in Implementing in VietnamDocument14 pagesSmart Locker - A Sustainable Urban Last-Mile Delivery Solution: Benefits and Challenges in Implementing in VietnamQuynh LeNo ratings yet
- Brock Planetary Declination SDocument6 pagesBrock Planetary Declination SDositheus Seth100% (2)
- Mercedez-Benz: The Best or NothingDocument7 pagesMercedez-Benz: The Best or NothingEstefania RenzaNo ratings yet
- 9446 - Data Sheets Final PDFDocument17 pages9446 - Data Sheets Final PDFmarounNo ratings yet
- Iloilo City Regulation Ordinance 2006-010Document4 pagesIloilo City Regulation Ordinance 2006-010Iloilo City CouncilNo ratings yet
- PDFDocument8 pagesPDFDocNo ratings yet
- +chapter 6 Binomial CoefficientsDocument34 pages+chapter 6 Binomial CoefficientsArash RastiNo ratings yet
- Primakuro Catalogue Preview16-MinDocument10 pagesPrimakuro Catalogue Preview16-MinElizabeth LukitoNo ratings yet
- Karan AsDocument3 pagesKaran AsHariNo ratings yet
- An Experimental Investigation On Abrasive Jet Machining by Erosion Abrasive GrainDocument3 pagesAn Experimental Investigation On Abrasive Jet Machining by Erosion Abrasive GrainPkNo ratings yet
- 1 28701-FGC+101+3441+Router+6471+Datasheet+Rev+FDocument2 pages1 28701-FGC+101+3441+Router+6471+Datasheet+Rev+FВладимир ЕгоровNo ratings yet
- The Sea DevilDocument6 pagesThe Sea DevilRevthi SankerNo ratings yet
- RA9275Document49 pagesRA9275znarf_ryanNo ratings yet
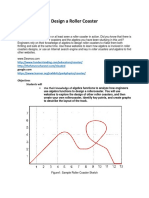
- Design A Roller Coaster ProjectDocument4 pagesDesign A Roller Coaster Projectapi-3564628400% (1)