Professional Documents
Culture Documents
Functional Group Interconversions Alcohols & The Carbonyl Group
Uploaded by
Kevin RadaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Functional Group Interconversions Alcohols & The Carbonyl Group
Uploaded by
Kevin RadaCopyright:
Available Formats
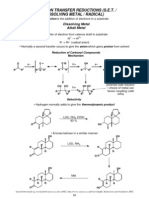
FUNCTIONAL GROUP INTERCONVERSIONS
ALCOHOLS & THE CARBONYL GROUP
INTRODUCTION
• So far we have discussed methods for the formation of the carbon skeleton
• In a large number of these reactions we found that either the starting material or the product
contained an alcohol or a carbonyl group
• Due to the importance of these two groups we will take a very brief look at them both
ALCOHOL OXIDATION
• Alcohols can readily be oxidised to the carbonyl moiety
• This is an incredibly important reaction as we have seen that the carbonyl group is one of the
cornerstones of C–C bond formation (organometallics, neutral nucleophiles, aldol, Julia,
Peterson & Wittig reactions)
R1 = H
OH O O
R R1 R R1 R OH
• Primary (R1 = H) alcohols – normally more reactive than seconary alcohols on steric grounds
• Need to be able to control oxidation of primary alcohols so only obtain aldehyde or acid
• Large number of reagents – all have their advantages and disadvantages
• Look at some of the more common... fragmentation common
to most oxidations (as
Chromium (VI) Oxidants you shall see)
Cr(VI) General Mechanism
Cr(IV)
O
OH2 proton O H
O H Cr
O Cr O
–H2O transfer HO OH
Cr Cr
O O R
O O O HO R HO
H
O R
• This fragmentation mechanism is common to most oxidations regardless of the nature of the
reagent
"Overoxidation" formation of carboxylic acids
• Invariably achieved in the prescence of H2O and proceeds via the hydrate
O
O
O H2O OH Cr O
O O Cr OH
O O
H
R H R R OH
OH H
R
OH
Jones Oxidation
H2SO 4, CrO3, acetone
OH O OH O
R H R OH R R1 R R1
• Harsh, acidic conditions limit use of this method
Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6. Strategy in Synthesis
1
Pyridinium Chlorochromate (PCC)
Cl
must avoid
water Cr O
O N
O H
OH O OH O
R H R H R R1 R R1
• Less acidic than Jones reagent (although still acidic)
Pyridinium Dichromate (PDC)
O O
O Cr O Cr O
O O N
H 2
• Even milder than PCC and has useful selectivity
O OH O
PDC PDC
R H DCM R H DMF R OH
Other Oxidants
Manganese Dioxide
MnO2
• Mild reagent
• Very selective – only oxidises allylic, benzylic or propargylic alcohols
HO HO
MnO2
only oxidises
activated alcohol
OH O
Activated Dimethylsulfoxide (DMSO) Oxidations
DMSO, activator & base
• Possibly the most widely used group of oxidants
• Huge number of variants depending on the nature of the activator or the base
• The most common is the Swern Oxidation
activator
DMSO
OH 1. Me2S(O), (COCl)2, DCM O
2. Et3N
• Mild (especially with wide choice of reagents)
• Overoxidation never a problem
• 1,2-Diols are not cleaved (see below)
Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6. Strategy in Synthesis
2
Mechanism
O O Cl
O O S
Cl S Cl
S S Cl O
O O R O
Cl H
common intermediate to all
activated DMSO oxidations
O S
O
S
H R O R
H R
H base: H
• Please note the similarity between this mechanism and the Cr(VI) mechanism
Cleavage of 1,2-Diols
• Many metal based oxidising agents will cleave 1,2-diols
• This can be synthetically useful reaction
• When it is desired NaIO4 or Pb(OAc)4 normally used
HO OH NaIO4 O O
R R1 R R1
O O
I proton transfer
O O
O OH O
O O O
O I proton transfer I
O OH O O
R R1 R R1
From Nicolaou's synthesis of amphoteronolide B
OH 1. (COCl)2, DMSO; then Et3N
BnO BnO CO2Me
O 2. Ph3P=CH2CO2Me O
CARBONYL REDUCTION
• Alcohols prevalent throughout pharmacologically interesting molecules
• A versatile method of introducing them is via carbonyl reduction
• Again not going into great detail just give you an overview of some of the more common
lithium activates Lithium Aluminium Hydride (LiAlH4 or LAH)
carbonyl
H3Al Li H3Al Al
Li O
H3Al O O O
H R R H R
R δ+ R
1
group 3 so H
R1
H
R1 R1
Lewis acid 4
number of repetitions
depends on sterics of the
carbonyl
• Each addition is slower
• Alkoxide electron-withdrawing group so reduces reactivity of hydride
Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6. Strategy in Synthesis
3
• Reduces most carbonyl functionality
• Little or no selectivity
• By altering the substituents on aluminium the reactivity can be tuned
• Bulky and electron withdrawing groups (alkoxides) reduce activity and make reagent more
selective
Sodium Borohydride (NaBH4)
• Considerably milder than LiAlH4
• Selectively reduces aldehydes and ketones in the presence of esters
only ketone will LiAlH4 would
react with NaBH4 reduce both
O O OH O
still reducing agent
OR OR but alkoxide reduces
reactivity
H 3B H R R
O H OEt OH EtOBH3
O R1 R1
H Et
• Not saying this is concerted (all occuring at once)
• Altering substituents on boron changes behaviour
• Add electron donating groups (alkyl) and increase the reactivity
NaBH4 vs LiAlH4
NaBH4
O O O O O
R H
> R R1
> R OR1
> R NR1 2
> R OH
LiAlH4
Diisobutylaluminium hydride (DIBAL)
• A good, strong reducing agent
• Different mechanism to the two previous metal-hydrides
• Aluminium centre is a Lewis acid and needs to coordinate to a Lewis base to activate hydride
• DIBAL = electrophilic reducing agent (e– rich carbonyls)
• NaBH 4 & LiAlH 4 = nucleophilic reducing agent (e– poor carbonyls)
coordination intramolecular
activates hydride R delivery
R AlR2
O Al O H OH
O H
Al R1
R R1 R H R
H R R1 R1
• Advantage of DIBAL is that reduction of esters can be stopped at alcohol or aldehyde
stable at low
temperature
AlR2
OH O O H O
2 x DIBAL 1 x DIBAL
R H R OR1 -78 ˚C R H R H
R1O
Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6. Strategy in Synthesis
4
From Corey's synthesis of the prostaglandins
O OH
OH
O O H
H H
1 x DIBAL CHO
-78 ˚C H
H H
RO R
RO R RO R
Borane (BH3)
• Like DIBAL, borane is an electrophilic reducing agent (e– rich carbonyls first)
• As a result reactivity is complete reverse of LiAlH 4 & NaBH4
O O O O O
R H R R1 R OR1 R NR1 2 R OH
LiAlH4
✓ ✓ ✓ ✓ ✗ /✓
NaBH4
✓ ✓ ✗ ✗ ✗
BH3
✗ /✓ ✗ /✓ ✗ /✓ ✓ ✓
Oxidation and Reduction
• The importance of these two operations is highlighted by the vast number of methods for
excuting both. You need to be aware that there are many examples reagents and catalysts that
can perform both diastereo and enantioselective reductions. There are also a number of
reagents that can perform selective oxidations via either kinetic resolution or
desymmetrisations.
FUNCTIONAL GROUP INTERCONVERSION: ACETAL FORMATION
• Last transformation for todays lecture combines alcohol and aldehyde / ketone
• You should have already met this...
Oxygen nucleophilies
• Add to carbonyls BUT they are also good leaving groups so reaction reversible
• Normally use large excess of nucleophile to drive reaction to completion
• Can stop at half way stage to form hemiacetals
O MeOH, H MeO OMe
H2O, H
• Reversibility of reaction useful as it means acetals can used as carbonyl protecting group
O O OMe O OMe OH O OH
MeOH R-MgBr H2O
H MeO R H R
OMe MeO OMe R R
aldehyde would be acetal inert
regenerate aldehyde
attacked by Grignard
Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6. Strategy in Synthesis
5
• It should be noted that if your compound is a diol it too can be protected as an acetal
O
OH OH O
O O O
R OMe H R OMe
Mechanism
H OH H
O H OH
O
O O
H Me Me
O Me
protonation increases polarisation of
hemiacetal
H
carbonyl considerably
provides another resonance form
OMe H Me OH2
MeO OMe O
O
Me O
Me
acetal O Me
H water a good leaving
group (stable, a lot of it
Nitrogen Nucleophiles about)
O R R H
RNH2 N N
+
– H2O imine enamine
Mechanism
proton transfer
O HNRR' O NHRR' H2O NRR'
loss of proton to
neutralise charge
R R' R R'
N N
base
H
enamine iminium
• Primary amines generally give imines
• Secondary amines generally give neutral enamines via the charged iminum species
• You have seen the use of enamines as enolate equivalents already
What have we learnt?
• A number of selective reagents for both oxidation and reduction
• Acetal formation is reversible
• As a result acetals make good protecting groups
• O, N and S nucelophiles can be used to form acetals
Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6. Strategy in Synthesis
6
You might also like
- Metal ReductionDocument11 pagesMetal Reductiondeepthimahanthi sabithaNo ratings yet
- Stabilitas Obat - OksidasiDocument46 pagesStabilitas Obat - OksidasiFadila FadilaNo ratings yet
- 생체의학공학 04 BiomaterialsDocument124 pages생체의학공학 04 Biomaterials생따일괄기No ratings yet
- Lec 5 Aldehyde Ketone Nucleophilic Addition PDFDocument78 pagesLec 5 Aldehyde Ketone Nucleophilic Addition PDFAssyakurNo ratings yet
- Pertemuan 5 Reguler Pagi & Sore 2021-2022 - 2Document53 pagesPertemuan 5 Reguler Pagi & Sore 2021-2022 - 2Amelia Dwi Ramadhani AjitiaNo ratings yet
- OzonolysisDocument2 pagesOzonolysisKamaraj NaiduNo ratings yet
- MitDocument13 pagesMitKasi RuddrarajuNo ratings yet
- Functional Group Reactions: C Synthesis Strategies, Chem 315/316 / Beauchamp 1Document19 pagesFunctional Group Reactions: C Synthesis Strategies, Chem 315/316 / Beauchamp 1Zia urRehman100% (1)
- Ch3e4 Stereoselective Synthesis MW Handout Reorganised 021111Document51 pagesCh3e4 Stereoselective Synthesis MW Handout Reorganised 021111Kethavath VenkateshNo ratings yet
- Carbohidratos Estructura PDFDocument6 pagesCarbohidratos Estructura PDFPatricia NarvaezNo ratings yet
- Module2 Reduction PDFDocument55 pagesModule2 Reduction PDFAnonymous vRpzQ2BLNo ratings yet
- Addition To CCDocument17 pagesAddition To CCHadiNo ratings yet
- Biochem2 Carbs and LipidsDocument69 pagesBiochem2 Carbs and Lipidstml_19672682No ratings yet
- Antibiotik Betha LaktamDocument9 pagesAntibiotik Betha LaktamdmujahidinNo ratings yet
- Glyc o SidesDocument22 pagesGlyc o Sidessiddra khalidNo ratings yet
- Biodegradable Polymers: Chemistry, Degradation and ApplicationsDocument26 pagesBiodegradable Polymers: Chemistry, Degradation and ApplicationsAnand GuptaNo ratings yet
- Natural PolymersDocument59 pagesNatural PolymersOlayanjuNo ratings yet
- Cheat Sheet For Organic Chemistry Midterm 1 2015 1Document1 pageCheat Sheet For Organic Chemistry Midterm 1 2015 1baba yagaNo ratings yet
- Chemistry 108B Exam #2 Cheat Sheet 2Document1 pageChemistry 108B Exam #2 Cheat Sheet 2雪郎かざきNo ratings yet
- Steroid-4697 VFGGHDDGBJDocument15 pagesSteroid-4697 VFGGHDDGBJEdy PurnomoNo ratings yet
- Ruthenium in Organic Synthesis 2004 - CruzDocument14 pagesRuthenium in Organic Synthesis 2004 - CruzswintarkaNo ratings yet
- DR - Modi-Sol-Gel Nano Coating22 - MANIT Dec.2015Document22 pagesDR - Modi-Sol-Gel Nano Coating22 - MANIT Dec.2015Deepen BanoriyaNo ratings yet
- Aldehydes Ketones and Acids ClassifiedDocument15 pagesAldehydes Ketones and Acids ClassifiedSsNo ratings yet
- Anhydride RxnsDocument1 pageAnhydride Rxnsapi-465421809No ratings yet
- 15.13 ThiolsDocument19 pages15.13 ThiolsSNo ratings yet
- Biodegradable Materials 1Document26 pagesBiodegradable Materials 1Louie Shaolin LungaoNo ratings yet
- Carbohydrate: ClassificationDocument67 pagesCarbohydrate: ClassificationKim Ryan Ello CagasNo ratings yet
- Ozonlysis - Only QPDocument3 pagesOzonlysis - Only QPAbhinav SinghNo ratings yet
- Week 13 WorkshopDocument3 pagesWeek 13 Workshoplayla_loveNo ratings yet
- Chemistry and Application of AntioxidantsDocument12 pagesChemistry and Application of Antioxidantsjd2604No ratings yet
- CHEM F311 Lecture 38 39 1,5-Dicarbonyl CompoundsDocument9 pagesCHEM F311 Lecture 38 39 1,5-Dicarbonyl Compoundsliving luxuriousNo ratings yet
- Retrosynthesis Approach to Organic Synthesis FGIsDocument28 pagesRetrosynthesis Approach to Organic Synthesis FGIsIvy JoyceNo ratings yet
- Mike Virnig - Crud PresentationDocument34 pagesMike Virnig - Crud Presentationworquera2507No ratings yet
- Chem 30BL Lecture 8a EsterificationDocument21 pagesChem 30BL Lecture 8a EsterificationKelly Llorin0% (1)
- Oxoacids of Chlorine by H To O ChemistryDocument44 pagesOxoacids of Chlorine by H To O ChemistryRitu JoharNo ratings yet
- Mechanisms 1-10: CHEM 725: Davey 1Document7 pagesMechanisms 1-10: CHEM 725: Davey 1Bradley DaveyNo ratings yet
- Named ReactionsDocument15 pagesNamed ReactionsSony mulgundNo ratings yet
- Table of K ValuesDocument7 pagesTable of K ValuesdasoodaseeNo ratings yet
- SOME KEY DETAILS ABOUT "NOVICHOK" NERVE AGENTS AND THE SECRET "FOLIANTDocument5 pagesSOME KEY DETAILS ABOUT "NOVICHOK" NERVE AGENTS AND THE SECRET "FOLIANTAlbertas VandenysNo ratings yet
- Synthesis of Commercial Drugs 2011-12 - M2 HanoiDocument28 pagesSynthesis of Commercial Drugs 2011-12 - M2 HanoiCy MoonNo ratings yet
- BPS 2110 F15 MetabolismDocument19 pagesBPS 2110 F15 MetabolismSumayah Al-SamiNo ratings yet
- Topic 4.8 Amino Acids Structure Acid-Base Properties Condensation Reactions ProteinsDocument8 pagesTopic 4.8 Amino Acids Structure Acid-Base Properties Condensation Reactions ProteinsSammyJayNo ratings yet
- Synthetic Reagents and Applications: 1.aluminium Isopropoxide 2.N-Bromosuccinimide 3.diazomethaneDocument19 pagesSynthetic Reagents and Applications: 1.aluminium Isopropoxide 2.N-Bromosuccinimide 3.diazomethaneHimanshu PanchalNo ratings yet
- IV Chemistry of Carbonyl CompoundsDocument29 pagesIV Chemistry of Carbonyl CompoundsK T Prajwal PrathikshNo ratings yet
- Lignan Profile of Piper Cubeba, An Indonesian Medicinal PlantDocument6 pagesLignan Profile of Piper Cubeba, An Indonesian Medicinal PlantRegiane Godoy de LimaNo ratings yet
- Copia de Aldehyde ReactionsDocument5 pagesCopia de Aldehyde Reactionsileanajaiseh26No ratings yet
- Roadmap - Main Corrected 2013Document1 pageRoadmap - Main Corrected 2013Luân Chu Nguyễn NhậtNo ratings yet
- Fisiologi TumbuhanDocument63 pagesFisiologi TumbuhanMasnawati WatiNo ratings yet
- OzonolysisDocument4 pagesOzonolysisRashi JalanNo ratings yet
- CarbohydrateDocument71 pagesCarbohydrateStevenson AgustinNo ratings yet
- Ca Rxns IDocument1 pageCa Rxns Iapi-465421809No ratings yet
- Organic Name Reactions: Nutshell Review & Preview ofDocument9 pagesOrganic Name Reactions: Nutshell Review & Preview ofSai YashwanthNo ratings yet
- Carbonyl ChemistryDocument63 pagesCarbonyl Chemistryelgendy1204100% (3)
- Bioorganic Chemistry and Biochemistry CHM3218 Summer C 2008: Class WebsiteDocument45 pagesBioorganic Chemistry and Biochemistry CHM3218 Summer C 2008: Class WebsiteMadhu MattaNo ratings yet
- Organic Chem NotesDocument49 pagesOrganic Chem NotesPriyaNo ratings yet
- Lecture #3Document10 pagesLecture #3waleejah maqboolNo ratings yet
- Doc-20170131-Wa0159 1 1Document9 pagesDoc-20170131-Wa0159 1 1rashidNo ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Ghee Chemistry: Karuna Meghwal Assistant Professor MIDFT, MehsanaDocument50 pagesGhee Chemistry: Karuna Meghwal Assistant Professor MIDFT, MehsanaNamraNo ratings yet
- Material Safety Data Sheet (PKE)Document8 pagesMaterial Safety Data Sheet (PKE)ffeedsupplierNo ratings yet
- On-Line Monitoring of Bacterial and Pathogen Load Through Adenosine TriphosphateDocument8 pagesOn-Line Monitoring of Bacterial and Pathogen Load Through Adenosine TriphosphateKhoiril NNo ratings yet
- Unit 4 Exam-SolutionsDocument7 pagesUnit 4 Exam-SolutionsbrunosipodNo ratings yet
- Unit-Ii Notes .Sme: ThermodynamicsDocument19 pagesUnit-Ii Notes .Sme: Thermodynamicssthavir punwatkarNo ratings yet
- Cell Communication Practice TestDocument5 pagesCell Communication Practice Testapi-237801056100% (2)
- Barazan® D Plus™: Viscosifier/Suspension AgentDocument1 pageBarazan® D Plus™: Viscosifier/Suspension AgentMarcoAntonioSerranoBazanNo ratings yet
- Duplex and Superduplex Stainless Steel Fittings (Amendments/Supplements To Astm A 815)Document11 pagesDuplex and Superduplex Stainless Steel Fittings (Amendments/Supplements To Astm A 815)Mathew CherianNo ratings yet
- Cascade Aeration Lecture 6 Environmental Engineering Unit Operations Spring 2014Document16 pagesCascade Aeration Lecture 6 Environmental Engineering Unit Operations Spring 2014Song Nguyen NguyenNo ratings yet
- Ix All Subj Guess PapersDocument27 pagesIx All Subj Guess PapersAsim AbbasNo ratings yet
- Hazard AnalysisDocument39 pagesHazard AnalysisvishnuNo ratings yet
- Spintronics Based Random Access Memory: A ReviewDocument19 pagesSpintronics Based Random Access Memory: A ReviewAgtc TandayNo ratings yet
- Consumables Accessories DMA 80 PDFDocument4 pagesConsumables Accessories DMA 80 PDFwillwNo ratings yet
- Definition of TermsDocument6 pagesDefinition of TermsFrancisco ZieNo ratings yet
- Iriotec - 8850 - Merck - TDS (For Rest of The World) PDFDocument2 pagesIriotec - 8850 - Merck - TDS (For Rest of The World) PDFxy2zjgNo ratings yet
- Carbonate Acidizing Design PDFDocument15 pagesCarbonate Acidizing Design PDFNelson PuentsNo ratings yet
- Quantum TunnellingDocument11 pagesQuantum Tunnellingrr1819100% (1)
- Coal Preparation Technologies Sep 2019 Indpnesia (JCOAL)Document23 pagesCoal Preparation Technologies Sep 2019 Indpnesia (JCOAL)ErwinLBudiNo ratings yet
- N ch3 07Document2 pagesN ch3 07yashNo ratings yet
- Aws-Practical Reference Guide PDFDocument34 pagesAws-Practical Reference Guide PDFpandimrNo ratings yet
- The Tensile-Yield Behavior ., of Ship'"S Ee.L: W: S. Owen, B, L Averbach and "Morris Coh&hDocument45 pagesThe Tensile-Yield Behavior ., of Ship'"S Ee.L: W: S. Owen, B, L Averbach and "Morris Coh&hKaung Myat HeinNo ratings yet
- Fluid Mechanics Tutorial on Fluid Properties and CalculationsDocument2 pagesFluid Mechanics Tutorial on Fluid Properties and CalculationsGabrielNo ratings yet
- Experiment 3 Biotransformation Reactions - Reduction of Carbonyls With Whole Plant PartsDocument4 pagesExperiment 3 Biotransformation Reactions - Reduction of Carbonyls With Whole Plant PartsTEN CHEANG100% (1)
- Extended Safety Data Sheet for Agidol-1Document24 pagesExtended Safety Data Sheet for Agidol-1Sekawan CosmeticsNo ratings yet
- DisinfectionDocument54 pagesDisinfectionShari KNo ratings yet
- Active AbsorptionDocument2 pagesActive AbsorptionANo ratings yet
- OzonolysisDocument1 pageOzonolysisThu NguyenNo ratings yet
- Protocols Cleaning Disinfection SterilizationDocument23 pagesProtocols Cleaning Disinfection SterilizationGeneSegoviaNo ratings yet
- Philippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationDocument1 pagePhilippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationFrancis SevillenoNo ratings yet
- Dielectric Constants Chart: How To Use This GuideDocument10 pagesDielectric Constants Chart: How To Use This GuideDewet VirmondNo ratings yet