Professional Documents
Culture Documents
VT Dalam Kehamilan
Uploaded by
Yosi W KusumaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
VT Dalam Kehamilan
Uploaded by
Yosi W KusumaCopyright:
Available Formats
Ventricular arrhythmias during
pregnancy
All topics are updated as new evidence becomes available and our peer
review process is complete.
INTRODUCTION — Arrhythmias are the most common cardiac
complication encountered during pregnancy in women with and without
structural heart disease [1-3]. Arrhythmias may manifest for the first time
during pregnancy or pregnancy can trigger exacerbations in women with
known pre-existing arrhythmias [1,4-6].
The prevalence, clinical presentation and management of ventricular
arrhythmias will be reviewed. Cardiac arrest during pregnancy, general
management of ventricular arrhythmias and cardiac arrest,
electrocardiographic characteristics of ventricular arrhythmias, and issues
relating to supraventricular arrhythmias during pregnancy are discussed in
detail elsewhere. (See "Cardiopulmonary arrest in pregnancy" and
"Approach to the diagnosis of wide QRS complex tachycardias" and
"Advanced cardiac life support (ACLS) in adults" and "ECG tutorial:
Ventricular arrhythmias" and "Supraventricular arrhythmias during
pregnancy" and "Wide QRS complex tachycardias: Approach to
management".)
GENERAL APPROACH — Women with established arrhythmias or
structural heart disease are at highest risk of developing arrhythmias
during pregnancy. Due to surgical advances, the number of women of
childbearing age with congenital heart disease has increased and this group
of women is at particularly high risk for arrhythmias (figure 1) [1,2,7-11]
(see "Pregnancy in women with congenital heart disease: General
principles"). Since arrhythmias are frequently associated with acquired or
structural heart disease, any woman who presents with an arrhythmia
during pregnancy should undergo clinical evaluation for structural heart
disease (including an electrocardiogram and a transthoracic
echocardiogram). (See 'VT in women with structural heart disease' below.)
In general, the approach to the treatment of arrhythmias in pregnancy is
similar to that in the non-pregnant patient. However, due to the theoretical
or known adverse effects of antiarrhythmic drugs on the fetus,
antiarrhythmic drugs are generally reserved for the treatment of
arrhythmias associated with significant symptoms or hemodynamic
compromise [12-14]. Treatment strategies during pregnancy are hampered
by the lack of randomized trials in this cohort of women. Choice of therapy,
for the most part, is based on limited data from animal studies, case
reports, observational studies, and clinical experience.
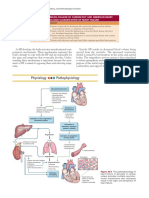
MECHANISM OF ARRHYTHMOGENESIS IN PREGNANCY — The
exact mechanism of increased arrhythmia burden during pregnancy is
unclear but has been attributed to hemodynamic, hormonal, and
autonomic changes related to pregnancy.
The hemodynamic changes of pregnancy have been well studied and these
changes likely contribute to the development of arrhythmias during
pregnancy [15,16]. Intravascular volume increases, augmenting the preload
on the ventricles, and increasing both atrial and ventricular size [15,17-20].
Atrial and ventricular myocardial stretch may contribute to
arrhythmogenesis due to stretch-activated ion channel activity causing
membrane depolarization, shortened refractoriness, slowed conduction,
and spatial dispersion of refractoriness and conduction [21-24]. There is
also an increase in resting heart rate that has been associated with markers
of arrhythmogenesis such as late potentials, premature ventricular
contractions, and depressed heart rate variability [25]. (See "Maternal
adaptations to pregnancy: Cardiovascular and hemodynamic changes".)
Few studies have been published on the influence of hormonal and
autonomic changes on arrhythmogenesis in pregnancy. Although
catecholamine levels do not appear to change during pregnancy, there is an
increase in adrenergic responsiveness during pregnancy [26-30]. Estrogen
has been shown to increase the number of myocardial alpha-adrenergic
receptors [31]. This increased adrenergic activity may contribute to
enhanced automaticity and triggered activity [32]. (See "Enhanced cardiac
automaticity".)
PALPITATIONS — Palpitations occur frequently during pregnancy and
are a common indication for cardiac evaluation during pregnancy. The
differential diagnosis of palpitations is extensive and the diagnostic
evaluation of pregnant women with palpitations does not differ from
nonpregnant women. (See "Overview of palpitations in adults".)
One study compared 110 pregnant women with symptoms suggestive of
possible arrhythmia (palpitations: 87 percent; dizziness: 13 percent;
syncope/presyncope: 6 percent) with 52 pregnant women evaluated for an
asymptomatic functional murmur [33]. Prevalence of supraventricular and
ventricular ectopic activity on 24-hour Holter ambulatory monitoring was
similar in the symptomatic and control groups.
Only 10 percent of symptomatic episodes were accompanied by the
presence of an arrhythmia [33]. A sensation of palpitations during
pregnancy in the absence of concomitant cardiac arrhythmias may be
related to the high output state, including increased heart rate, decreased
peripheral resistance, and increased stroke volumes. (See "Maternal
adaptations to pregnancy: Cardiovascular and hemodynamic changes".)
VENTRICULAR PREMATURE BEATS — Ventricular premature beats
(VPBs) are frequently detected in pregnant women. The prevalence is
dependent upon the duration of observation and the clinical presentation.
(See "Ventricular premature beats".)
In the above referenced study of 110 symptomatic and 52 asymptomatic
pregnant women, the prevalence of isolated VPBs was similar in
symptomatic and asymptomatic women (49 versus 40 percent) [33].
However, frequent VPBs (≥50 VPBs per 24 hours) were more common in
symptomatic women (22 versus 4 percent). There was a significant
reduction in the frequency of combined atrial and ventricular ectopic
activity in the nine women in whom Holter monitoring was repeated
postpartum.
Clinical presentation — VPBs produce few or no symptoms in the
majority of women, although some women may experience symptoms of
palpitations or dizziness. (See "Ventricular premature beats", section on
'Clinical manifestations'.)
Management during pregnancy — No therapy is required for VPBs in
asymptomatic patients. Pregnant women with symptomatic VPBs should
be reassured of the benign nature of VPBs.
Some experts counsel patients with palpitations to discontinue potential
precipitant factors such as smoking, coffee intake, alcohol intake, and other
stimulants [14]. However the role of caffeine restriction has not been
established. Moderate caffeine exposure has not been demonstrated to
increase VPBs in patients with or without structural heart disease [34,35],
and caffeine restriction was not found to improve symptoms or reduce the
frequency of VPBs in a small trial [36].
If ectopic activity continues and is associated with intolerable symptoms,
beta blockers can be used. Metoprolol is a preferred beta blocker in
pregnant women since atenolol may impair fetal growth. The limited
options for treatment of VPBs are discussed further separately. (See
"Ventricular premature beats", section on 'Treatment'.)
EPIDEMIOLOGY OF VT AND VF — Ventricular tachyarrhythmias
(ventricular tachycardia [VT] or ventricular fibrillation [VF]) are rare
during pregnancy [37]. VT can be seen in pregnant women without
apparent structural heart disease [38-40], but is often associated with
structural heart disease. The risk of recurrent VT during pregnancy is
particularly high (27 percent) in women with structural heart disease and a
history of VT [6]. Types of structural heart disease associated with VT in
pregnancy include hypertrophic cardiomyopathy [1,6,41,42], peripartum
cardiomyopathy [43,44], arrhythmogenic right ventricular cardiomyopathy
[45-50], congenital heart disease [1,6,51-53], and valvular heart disease [1].
Myocardial infarction with or without coronary artery disease has been
observed during pregnancy [54-59] and may be complicated by VT or VF
[57,58]. Women with primary electrical diseases such as long QT syndrome
[60,61] and Brugada syndrome [62] are also at risk of VT. (See
"Hypertrophic cardiomyopathy: Assessment and management of
ventricular arrhythmias and sudden cardiac death risk" and "Peripartum
cardiomyopathy: Etiology, clinical manifestations, and diagnosis" and
"Pregnancy in women with congenital heart disease: Specific lesions" and
"Clinical features and treatment of ventricular arrhythmias during acute
myocardial infarction" and "Congenital long QT syndrome: Epidemiology
and clinical manifestations" and "Arrhythmogenic right ventricular
cardiomyopathy: Anatomy, histology, and clinical manifestations" and
"Brugada syndrome: Clinical presentation, diagnosis, and evaluation" and
"Hypertrophic cardiomyopathy: Medical therapy", section on 'HCM during
pregnancy and delivery'.)
Other medical conditions associated with VT during pregnancy are
hypomagnesemia [63-65], hypertensive crises [66,67], and thyrotoxicosis
[68]. (See "Significance of hypomagnesemia in cardiovascular disease" and
"Gestational hypertension" and "Overview of thyroid disease in pregnancy"
and "Cardiovascular effects of hyperthyroidism".)
The management of ventricular arrhythmias needs to be tailored to the
individual. The following are among the clinical factors that should be
considered:
●Presence and severity of underlying heart disease
●Ventricular function
●Etiology of the VT (catecholamine sensitive versus noncatecholamine
sensitive)
●Frequency and duration of VT (nonsustained versus sustained)
●Severity of associated symptoms
The following section will review issues regarding VT during pregnancy in
women with and without structural heart disease.
IDIOPATHIC VT — Monomorphic ventricular tachycardia (VT) without
apparent structural heart disease is considered idiopathic. The most
common type of idiopathic VT is repetitive monomorphic VT, which
usually originates from the right ventricular outflow tract
(electrocardiogram [ECG] "signature": left bundle branch block and
inferior axis) or, less often, from the left ventricular outflow tract (ECG
pattern: right bundle branch block and inferior axis or left bundle and
inferior axis but earlier precordial transition than for right ventricular
outflow tract tachycardia). Another type is idiopathic left ventricular
tachycardia, which originates from the inferior aspect of the midseptum
and has the morphologic pattern of right bundle branch block with left axis
deviation (QRS axis around -60°). (See "Monomorphic ventricular
tachycardia in the absence of apparent structural heart disease" and
"Nonsustained VT in the absence of apparent structural heart disease".)
A study of seven women presenting with new-onset idiopathic VT during
pregnancy found that the VT was often catecholamine sensitive and that
the VT was often suppressed in women who received beta blockers [39].
There were no maternal or fetal complications in this series. There is one
case report of sudden death in a woman with idiopathic VT who died in the
third trimester, three weeks after initiation of procainamide therapy [69].
Management during pregnancy — Idiopathic VT rarely degenerates
into an unstable rhythm and usually has a benign prognosis [39,70].
●Treatment of repetitive monomorphic VT with cardioselective beta
blockers may be effective in pregnant women with idiopathic VT even in
the absence of a clear relationship to adrenergic tone [39,70-74]. Sotalol
can be used as an alternative [75]. (See "Monomorphic ventricular
tachycardia in the absence of apparent structural heart disease", section on
'Treatment of RMVT'.)
●The less common idiopathic left ventricular tachycardia appears to
respond well to verapamil, both for the termination of acute episodes and
the prevention of recurrences [76,77]. (See "Monomorphic ventricular
tachycardia in the absence of apparent structural heart disease", section on
'Treatment of ILVT'.)
LONG QT SYNDROME — Although ventricular tachycardia (VT) during
pregnancy has been reported in women with long QT syndrome (LQTS)
[78], the increase in heart rate seen during pregnancy may serve to shorten
the QT interval and therefore may be partially protective. (See "Congenital
long QT syndrome: Epidemiology and clinical manifestations".)
Risk during pregnancy — In women with LQTS, the risk of VT is
especially high in the postpartum period [61]. Increased risk of VT during
the postpartum period may be related to a decrease in the heart rate and an
associated increase in the QT interval. The physiologic stress and altered
sleep patterns associated with caring for a newborn infant may also
contribute to an increase in adrenergically mediated cardiac events.
The effect of pregnancy was evaluated in a retrospective analysis of 422
women (111 probands and 311 first-degree relatives) entered into the
International LQTS registry [61]. Most of the probands had a personal
history of syncope or aborted cardiac arrest. The following findings were
noted:
●Probands were significantly more likely to have cardiac events (syncope,
aborted cardiac arrest, or sudden cardiac death) in the 40-week
postpartum interval than during the prepregnancy period of 40 weeks
(23.4 versus 3.8 percent). The increase in risk was distributed throughout
the postpartum period. The incidence of first cardiac events during
pregnancy was slightly but not significantly increased compared with the
prepregnancy period (9.0 versus 3.8 percent).
●The postpartum increase in risk also applied to first cardiac events (9.0
versus 1.8 and 0 percent during and before pregnancy).
●Treatment with beta blockers was independently associated with a
decrease in risk for cardiac events in probands during all three intervals
(odds ratio 0.023).
●The average probability of having a cardiac event during the postpartum
period in probands was 2 percent (1 in 50 pregnancies). Treatment with a
beta blocker lowered the risk to 1 in 2500 pregnancies.
The risks associated with pregnancy may be different among various LQTS
genotypes (see "Congenital long QT syndrome: Epidemiology and clinical
manifestations", section on 'Influence of genotype on triggers'). The
influence of genotype is illustrated by the following observations:
●In a series of 388 LQTS patients referred for genetic testing, postpartum
cardiac events were more commonly reported in patients with LQT2
mutation (13 of 80, 16 percent) than in patients with LQT1 (1 of 103, <1
percent). [79].
●In a series limited to women with a single LQT1 mutation, cardiac event
rates associated with pregnancy were low (2.6 percent) [80]. These events
occurred only in women with a prior history of symptoms who were not
taking beta blockers.
Management during pregnancy — We agree with the 2006 American
College of Cardiology/American Heart Association/European Society of
Cardiology guidelines for management of patients with ventricular
arrhythmias and the prevention of sudden cardiac death and the 2011
European Society of Cardiology guidelines on the management of
cardiovascular diseases during pregnancy. The guidelines recommended
that pregnant women with LQTS who have had symptoms benefit from
continued beta blocker therapy throughout pregnancy and postpartum,
unless there are definite contraindications [75,81].
BRUGADA SYNDROME — There are limited reports of pregnancies in
women with Brugada syndrome. In one retrospective single-center study
including 104 women (219 deliveries) with Brugada syndrome, six women
(6 percent) experienced recurrent syncope during pregnancy [82]. Five
women continued to experience syncope after delivery and four women
received an implantable cardioverter-defibrillator (ICD), as syncope is
regarded a high-risk feature for ventricular arrhythmias. No serious events
were reported during the peripartum period. In three women with Brugada
syndrome and an ICD, no noteworthy problems were reported during
pregnancy. There is one case report of electrical storm during pregnancy
[62]. The use of low-dose isoproterenol infusion followed by oral quinidine
has been used to treat ventricular tachycardia and normalize the
electrocardiogram [62]. (See "Brugada syndrome: Prognosis, management,
and approach to screening", section on 'Treatment'.)
VT IN WOMEN WITH STRUCTURAL HEART DISEASE
Hypertrophic cardiomyopathy — In general, women with
hypertrophic cardiomyopathy (HCM) tolerate pregnancy well [42,83],
although several case reports have described cardiac complications and
sudden death during pregnancy [41,42,84-86]. (See "Hypertrophic
cardiomyopathy: Assessment and management of ventricular arrhythmias
and sudden cardiac death risk" and "Hypertrophic cardiomyopathy:
Medical therapy", section on 'HCM during pregnancy and delivery'.)
The largest study investigating mortality and morbidity in pregnant women
with HCM included 100 women who had a total of 199 live births [42]. Two
sudden cardiac deaths occurred during pregnancy in women with high-risk
features. One of the women had severe left ventricular hypertrophy (30
mm maximal wall thickness) and a resting outflow gradient of 115 mmHg.
She died suddenly four days after delivery after complaining of
palpitations. The other woman had a family history of eight deaths in
young relatives, five of which were sudden. This patient developed
recurrent episodes of sustained ventricular tachycardia (VT) during labor.
Management of pregnancy and delivery in women with HCM is discussed
separately. (See "Hypertrophic cardiomyopathy: Medical therapy", section
on 'HCM during pregnancy and delivery'.)
Congenital heart disease — The prevalence of sustained VT during
pregnancy in women with congenital heart disease (CHD) has been
reported to range from 4.5 to 15.9 per 1000 pregnancies [1,51] (see
"Pregnancy in women with congenital heart disease: General principles"
and "Pregnancy in women with congenital heart disease: Specific lesions").
Prevalence rates are strongly influenced by the types of cardiac lesions in
the study population.
●In a large prospective multicenter study in women with CHD, two cases of
sustained VT occurred in 445 pregnancies. One woman had an unrepaired
intracardiac shunt and the other had repaired congenital aortic stenosis
[1].
●A multicenter study from Japan reported two cases of sustained VT
during 126 pregnancies [51]. Both cases of VT occurred in women with
repaired tetralogy of Fallot and both were successfully treated with
intravenous lidocaine. Seven pregnancies were complicated by
nonsustained VT, none of which were treated.
Peripartum cardiomyopathy — Peripartum cardiomyopathy is a rare
and sometimes life-threatening condition defined as development of
systolic heart failure in the last month of pregnancy or within five months
of delivery (see "Peripartum cardiomyopathy: Etiology, clinical
manifestations, and diagnosis"). The incidence varies widely among
various populations. The clinical presentation includes symptoms of new-
onset heart failure such as dyspnea, cough, orthopnea, and hemoptysis.
The prevalence of sustained VT in patients with peripartum
cardiomyopathy is unknown; few cases have been reported in the literature
[43,44,87]. These ventricular arrhythmias can be refractory to
pharmacologic treatment (eg, lidocaine, metoprolol, amiodarone) and
direct current cardioversion [43,44,87].
Arrhythmogenic right ventricular cardiomyopathy — Most
pregnancies in women with arrhythmogenic
right ventricular cardiomyopathy are tolerated well [45-50]. Pregnancies
were managed successfully by close monitoring and antiarrhythmic drugs
when necessary. Data from the combined Johns Hopkins/Dutch ARVD/C
registry provided information on 26 women during 39 pregnancies (>13
weeks) [88]. A single episode of sustained ventricular arrhythmia
complicated five pregnancies (13 percent) of five women without a prior
history of sustained ventricular arrhythmias. Interruption of beta blockers
was associated with two of these events. Previous studies have shown that
withdrawal of beta blockers during pregnancy may exacerbate the
occurrence of VT during pregnancy [47,50].
MANAGEMENT OF VT DURING PREGNANCY IN WOMEN
WITH STRUCTURAL HEART DISEASE
Management of acute episodes — Acute treatment of sustained
ventricular arrhythmias in pregnant women is similar to that in
nonpregnant women. Ventricular arrhythmias in the presence of structural
heart disease are potentially life-threatening and require immediate
evaluation for hemodynamic instability to determine whether electrical
cardioversion or defibrillation is indicated [75,81].
In hemodynamically well-tolerated ventricular tachycardia (VT),
pharmacological cardioversion may be acceptable. Pharmacological
options include intravenous procainamide, amiodarone, or lidocaine
[75,81]; the choice of pharmacological agents should be tailored to the
individual case. For women at risk for VT during labor and delivery, it is
important to ensure that appropriate cardiac medications and external
defibrillators are available in the delivery suites.
Electrical cardioversion — Emergent or elective electrical
cardioversion can be performed at all stages of pregnancy [14,89-95].
Electrical cardioversion is indicated for any sustained VT with
hemodynamic compromise [75,81] and can be considered for drug-
refractory VT (see "Basic principles and technique of electrical
cardioversion and defibrillation" and "Cardioversion for specific
arrhythmias"). Electrical cardioversion does not result in compromise of
blood flow to the fetus [96]. While there is a theoretical risk of inducing an
arrhythmia in the fetus, this risk is very small due to the high fibrillation
threshold and small amount of energy reaching the fetus [14,89,97].
Nonetheless, fetal rhythm monitoring is recommended because of rare
reported cases of cardioversion precipitating fetal distress requiring
emergency cesarean delivery [98]. In the third trimester, some physicians
prefer to perform electrical cardioversion under general anesthesia and
intubation considering the more difficult airway and increased risk of
gastric aspiration during pregnancy.
Prophylactic pharmacologic therapy during pregnancy — The risk
of recurrent VT and sudden death in women with structural heart disease
can be substantial and the benefits of prophylactic drug therapy may
outweigh the potential fetal adverse effects of these drugs (see "Overview of
sudden cardiac arrest and sudden cardiac death"). The risk of sudden
death is further increased when concomitant left ventricular dysfunction is
present.
Depending on the underlying cardiac condition, beta-1 selective beta-
blockers alone, amiodarone alone, or both in combination can be effective
in preventing VT during pregnancy as noted in the 2006 American College
of Cardiology/American Heart Disease/European Society of Cardiology
ventricular arrhythmia guidelines [75]. Gestational exposure to
amiodarone is associated with neonatal hypothyroidism and
hyperthyroidism. Small-for-gestational-age infants are reported with
gestational exposure to the combination of amiodarone and beta blockers
[99]. In some cases, sotalol can be considered if beta blocker therapy is
ineffective. Because of potential side effects, all women should be
counseled about the potential risks and benefits of drug therapy. (See
"Amiodarone: Monitoring and management of side effects" and "Clinical
uses of amiodarone".)
Although some have used class IA (eg, quinidine, procainamide) or IC (eg,
flecainide) drugs as prophylactic treatment for VT during pregnancy
[100,101], these drugs are not generally recommended since they have not
improved survival in the nonpregnant population with structural heart
disease, presumably because of proarrhythmic effects [102].
Implantable cardioverter-defibrillator — Women with an
implantable cardioverter-defibrillator (ICD) can have a successful
pregnancy with good fetal outcome [103-105]. Indications for ICD
placement are discussed separately. (See "Secondary prevention of sudden
cardiac death in heart failure and cardiomyopathy".)
In a retrospective multicenter study of pregnancy outcomes in women with
ICDs (n = 44), 25 percent (11 of 44) of the pregnancies were complicated by
at least one shock [103]. All ICDs were implanted for secondary prevention
and the underlying cardiac diseases were primary electrical diseases (ie,
long QT syndrome, idiopathic ventricular fibrillation) or structural heart
diseases (ie, cardiomyopathy, congenital heart disease, arrhythmogenic
right ventricular cardiomyopathy). Pregnancy was not associated with an
increase in ICD-related complications or an increase in the number of
shocks (0.07 versus 0.06 shocks per month) compared with the
preconception period [103].
The experience with ICD implantation during pregnancy is limited [106];
however, pacemaker implantation during pregnancy can be accomplished
and total radiation dose can be reduced by using echocardiographic
guidance [107,108].
If a pregnant patient is determined to be at high risk of sudden death,
placement of an implantable defibrillator is generally deferred until after
delivery of the baby. In this setting, an external wearable automatic
defibrillator (LifeVest) is often prescribed for the duration of the
pregnancy.
Radiofrequency catheter ablation — The success rate of
radiofrequency catheter ablation of monomorphic VT is between 80 to 100
percent [109-113] and may be considered in women who are using
antiarrhythmic therapy and are contemplating pregnancy (see "Overview
of catheter ablation of cardiac arrhythmias"). Currently, there is only
limited experience with catheter ablation of VT during pregnancy [114].
Experience with radiofrequency catheter ablation during pregnancy has
been limited to cases of SVT [115-122]. These procedures are generally not
performed during pregnancy, mainly due to concerns of ionizing radiation
exposure to the fetus. However, in rare cases, women with severe and drug-
resistant VT during pregnancy may be considered for an ablation
procedure. The risk of radiation exposure for the fetus during a typical
ablation is small (<1 mGy at all periods of gestation) and is mainly
attributable to scatter from the thorax of the mother [123]. (See
"Diagnostic imaging procedures during pregnancy", section on 'Effects of
ionizing radiation on the fetus'.)
ISSUES REGARDING ANTIARRHYTHMIC DRUG
TREATMENT — Use of antiarrhythmic drugs in pregnancy requires
attention to potential alterations in pharmacokinetics as well as fetal risk.
For most antiarrhythmic drugs, adequate and well-controlled studies in
pregnant women are lacking and most drugs are categorized as class C
drugs by the US Food and Drug Administration (table 1 and table 2)
[124,125]. Another consideration is potential adverse effects in the infant
during breast feeding (table 2). Antiarrhythmic drug safety during
pregnancy, teratogenic risk, pharmacokinetic changes, and breast feeding
are discussed further separately. (See "Supraventricular arrhythmias
during pregnancy", section on 'Issues regarding antiarrhythmic drug
treatment'.)
SOCIETY GUIDELINE LINKS — Links to society and government-
sponsored guidelines from selected countries and regions around the world
are provided separately. (See "Society guideline links: Arrhythmias in
adults" and "Society guideline links: Ventricular arrhythmias".)
SUMMARY AND RECOMMENDATIONS
●Ventricular tachyarrhythmias are frequently associated with acquired or
structural heart disease and therefore any woman who presents with a
ventricular arrhythmia during pregnancy should undergo clinical
evaluation for structural heart disease (including an electrocardiogram and
a transthoracic echocardiogram). (See 'General approach' above and 'VT in
women with structural heart disease' above.)
●Monomorphic ventricular tachycardia without apparent structural heart
disease is considered idiopathic. The most common type originates from
the right ventricular outflow tract, and this form of ventricular tachycardia
can often be successfully treated with beta blockers or verapamil. (See
'Idiopathic VT' above.)
●Women with the long QT syndrome are at risk for ventricular
tachycardia, especially in the postpartum period. Pregnant women with
long QT syndrome should be treated with beta blocker therapy throughout
pregnancy and postpartum. (See 'Long QT syndrome' above.)
●Acute treatment of sustained ventricular arrhythmias in pregnant women
is similar to that in nonpregnant women. Ventricular arrhythmias in the
presence of structural heart disease are potentially life-threatening and
require immediate evaluation for hemodynamic instability to determine
whether electrical cardioversion or defibrillation is indicated. (See
'Management of acute episodes' above.)
●In women with structural heart disease and a history of ventricular
tachycardia, the benefits of prophylactic drug therapy may outweigh the
potential fetal adverse effects of these drugs. (See 'Prophylactic
pharmacologic therapy during pregnancy' above.)
●Women with an implantable cardioverter-defibrillator can have a
successful pregnancy with good fetal outcome. (See 'Implantable
cardioverter-defibrillator' above.)
●Information on the use of specific antiarrhythmic drugs in pregnancy,
including the US Food and Drug Administration risk category and
pregnancy implications, is available in the UpToDate drug database. (See
'Issues regarding antiarrhythmic drug treatment' above.)
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Makhoul2013 2Document5 pagesMakhoul2013 2Yosi W KusumaNo ratings yet
- Laboratory Test of Immune SystemDocument30 pagesLaboratory Test of Immune SystemYosi W KusumaNo ratings yet
- Forensic SerologyDocument46 pagesForensic SerologyYosi W KusumaNo ratings yet
- German Problems 2014Document152 pagesGerman Problems 2014Yosi W KusumaNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Echocardiography in TAVI ProcedureDocument26 pagesEchocardiography in TAVI ProcedurejshNo ratings yet
- Jurnal 4 Uji Jalan 6 MenitDocument10 pagesJurnal 4 Uji Jalan 6 MenitSandy Tama Okvan YudhaNo ratings yet
- Anatomy and PhysiologyDocument3 pagesAnatomy and PhysiologyChristopher bruncanoNo ratings yet
- Management Algorithm For Adult With HyperkalemiaDocument1 pageManagement Algorithm For Adult With HyperkalemiaAnjiNo ratings yet
- CardiomyopathyDocument23 pagesCardiomyopathyDefyna Dwi LestariNo ratings yet
- Patient Story: Life's Scond Chance After Cardiac SurgeryDocument2 pagesPatient Story: Life's Scond Chance After Cardiac SurgeryDel Marquez CusayNo ratings yet
- Acute Kidney Injury in Children: Being AWAREDocument4 pagesAcute Kidney Injury in Children: Being AWAREIrkania PasangkaNo ratings yet
- Relacion Colesterol Total A HDL y Colesterol No HD PDFDocument9 pagesRelacion Colesterol Total A HDL y Colesterol No HD PDFJoseAbdalaNo ratings yet
- Internal Medicine PDFDocument76 pagesInternal Medicine PDFroxxanaNo ratings yet
- Previous Question Paper 2 Cardiology PDFDocument6 pagesPrevious Question Paper 2 Cardiology PDFDeepthi D100% (1)
- Acs OsceDocument4 pagesAcs OsceYohanes ArviNo ratings yet
- ECG Interpretation Cheat SheetDocument14 pagesECG Interpretation Cheat Sheetrenet_alexandre75% (4)
- Catheter Ablation in End Stage Heart FailureDocument10 pagesCatheter Ablation in End Stage Heart FailureJose Gomez MorenoNo ratings yet
- Renal Emergency RevisiDocument103 pagesRenal Emergency Revisidesy f sarahNo ratings yet
- BRADIKARDIDocument26 pagesBRADIKARDIRichard 151289No ratings yet
- VSD 180207074930Document33 pagesVSD 180207074930widyaNo ratings yet
- Quantification of Severity of Mitral Regurgitation With The New ASE GuidelinesDocument20 pagesQuantification of Severity of Mitral Regurgitation With The New ASE GuidelinesPanfilAlinaNo ratings yet
- 2007 Electrocardiographic Manifestations Pediatric EcgDocument10 pages2007 Electrocardiographic Manifestations Pediatric EcgAngel NspNo ratings yet
- Cardiac ArrhythmiasDocument14 pagesCardiac ArrhythmiasArvin John ManuelNo ratings yet
- List of Hospitals Jaipur - 2Document17 pagesList of Hospitals Jaipur - 2Global Health Alliance FoundationNo ratings yet
- CCU AuditDocument75 pagesCCU AuditNica Reyes-Sindiong0% (1)
- Basic ECG For Refresher Course 2014Document116 pagesBasic ECG For Refresher Course 2014Winz DolleteNo ratings yet
- Differential Diagnosis of Glomerular DiseasesDocument2 pagesDifferential Diagnosis of Glomerular DiseasesMaryam Fadah100% (1)
- F4AECCF9 - PBL Week 4 KidneyDocument13 pagesF4AECCF9 - PBL Week 4 KidneyKahfi AzzumardiNo ratings yet
- Brunner and Suddarth's Textbook of Medical-Surgical Nursing 12th Ed. (Dragged) 4Document1 pageBrunner and Suddarth's Textbook of Medical-Surgical Nursing 12th Ed. (Dragged) 4jamie carpioNo ratings yet
- Seminar Electrocardiogram (ECG)Document32 pagesSeminar Electrocardiogram (ECG)Jamuna PatelNo ratings yet
- Heart Failure Treatment Algorithm: Diagnosis and ClassificationDocument7 pagesHeart Failure Treatment Algorithm: Diagnosis and ClassificationLorreine Elisa FaruqNo ratings yet
- The Ehra Book of Intervntional Electrophysiology - OxfordDocument321 pagesThe Ehra Book of Intervntional Electrophysiology - Oxfordmorris njageNo ratings yet
- Queensland RMO Application GuideDocument18 pagesQueensland RMO Application GuideSOMANATHAN UMAHSUTHANNo ratings yet
- PMN 310 Anti-Anginal Drugs: by Manasseh Mvula Bsc. N Iii StudentDocument54 pagesPMN 310 Anti-Anginal Drugs: by Manasseh Mvula Bsc. N Iii StudentManasseh MvulaNo ratings yet