Professional Documents
Culture Documents
Ac1 Forensics Itsinthecards
Uploaded by
Josh POriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ac1 Forensics Itsinthecards
Uploaded by
Josh PCopyright:
Available Formats
F Activity 1: It’s in the Cards
Deductive Reasoning & The PT
Forensic scientists and detectives, like those you saw
on Cold Case Files., must be able to organize, analyze,
and determine connections between a hodgepodge of
facts, physical evidence, personal statements, and prior
experiences. As a crime solver like those presented on
Cold Case Files, you not only have to be able to work
with this jumble of evidence, but you have to work
backwards and forwards in order to come to a logical
conclusion about a case. In this exercise, though, we’ll
work on just the experience of the detectives. You’ll
need to use facts, physical evidence, and your prior
experiences in this course to solve a puzzle of sorts.
With that in mind, today you’ll work with a chemistry
puzzle. You’ll use some of that periodic knowledge and
your deductive skills to better piece together all of the
aspects of periodic law.
Fig 1.1
P
P R E P A R I N G
WHAT DO YOU THINK? • LE ARNING OBJECTIVES
It is time to rack your brain a bit. You need to remember and use what you learned in the previous unit to
solve this puzzle. This is the first of many times that you’ll use what you’ve already learned, so if you are
still struggling with the last unit’s material, you need to get in for some help! For this activity, you’ll just
need selective memory. For each of the terms below, state in your own words what it is, and do your
best to draw a picture (at the nano scale) to illustrate each individual concept.
1. Electronegativity 4. Density
2. Atomic radius 5. Melting point (physical science)
3. Ionization energy 6. Oxides
As always, include an objective or essential question for this laboratory activity and share that objective
with a teammate or laboratory partner.
Based on Flinn Chem Topics
1
E
E X P E R I M E N T I N G
CRE ATING ORDER • PREDICTIONS
Ionization Atomic
Being Mendeleev… Energy Radius
You have a deck of cards in front of you. Each card, corresponding Specific Gravity Formula of its
to an individual element, has a set of data on it as represented in oxide XbOc
Figure 1.2. Your task is twofold: Formula of its
chloride XCla Melting Point
1. Create order by looking for trends, similarities, and
difference in each of the properties.
Density* Formula of its
2. Do some detective work and predict the properties of the hydride XHd
“Missing Element.” Just as Mendeleev didn’t have a full set
of elements to work with in his game of chemical solitaire Electronegativity
(the creation of the first periodic table), you too aren’t
working with a full deck (phew, I crack myself up). There *Density values are in units of g/cm3 for solids
should be a space in your order for the “Missing Element” and liquids, g/L for gases Fig 1.2
card.
3. When you are finished, call your instructor over to check your order. At that time you will receive
a key with one possible ordering of the elements based on the properties you have here.
You may NOT use any additional references to do this – so a periodic table, your text, other texts, the
Internet, the CRC, and other possible references are out! It is just you, your group, and your elemental
evidence to get this done. In your notebook, draw your final organizational chart representing a few of the
properties (so you could recreate these if necessary).
A
A N A L Y Z I N G
RE ADING • QUESTIONS
Reading: It’s in the Cards
,
Read Active Chemistry “Chem Talk” on 594 and 596 on deductive reasoning and the elements.
Questions: It’s in the Cards
1. Mendeleev’s periodic law can be stated: “The physical and chemical properties of elements are
periodic functions of their atomic masses.” Based on your layout of cards, define “periodic
functions.”
2. The current model of the periodic table is arranged by atomic number. Why didn’t Mendeleev
arrange the periodic table by atomic number?
3. Determine the atomic number and identity of each element. It might help to know that “specific
gravity” is actually atomic mass! You do not need to record anything for this step in your
laboratory notebook (just do it on the handout).
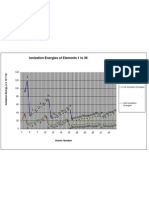
4. Using a program of your choice (or graph paper), create three graphs, graphing the ionization
energy, atomic mass, or electronegativity on the y-axis and atomic number on the x-axis. After
looking at the graphs, which property is not a periodic function? Explain.
Based on Flinn Chem Topics
2
5. There are certain trends which occur either across periods or down groups (families). For the
three properties in Figure 1.3, write if the trend is to increase or decrease as you go across a
period or down a group.
Fig 1.3
6. Be sure you can identify and label the following on the answer key passed out in class – alkali
metals, noble gases, f-block elements, alkali earth metals, halogens, transition metals,
metalloids. You do not need to write anything for this in your laboratory notebook, but you
should use this vocabulary on a regular basis.
7. What is the missing element? Predict its properties based on its group & the trends.
C R I T I C A L L Y T H I N K I N G
CT ME AN? • KNOW? • BELIEVE? • CARE?
What does the activity mean?
Chemistry explains the macroscopic phenomenon (what you observe) with and explanation of what
happens at the nanoscopic level (atoms and molecules) using symbolic structures as a way to
communicate. Explain the meaning of this activity by completing the MNS table.
MACRO NANO SYMBOLIC
How did you see periodic law? What is periodic law a function Symbolically we represent all
of at the nanoscale? of these trends & periodic law
with the periodic table! It is the
big symbol for this activity.
How do I know?
How do you know which properties are periodic trends and which are not?
Why do I believe?
This is your first time to cite references from a past unit. Name two activities this lab specifically
connects to from the Periodic Table unit? What is that connection?
Why do I care?
Why do you think knowing about the properties of the elements is crucial to a forensic scientist?
Based on Flinn Chem Topics
3
You might also like
- H 0910Document5 pagesH 0910Josh PNo ratings yet
- Ac1 Dominoes Molecules 0910Document3 pagesAc1 Dominoes Molecules 0910Josh PNo ratings yet
- Ac9 Forensics ChromatorgraphyDocument5 pagesAc9 Forensics ChromatorgraphyJosh PNo ratings yet
- Producing A Gas: Activity 2: Alternative PathwaysDocument5 pagesProducing A Gas: Activity 2: Alternative PathwaysJosh PNo ratings yet
- Activity 8: Metal Identification Metal Activity Series: Prep AringDocument6 pagesActivity 8: Metal Identification Metal Activity Series: Prep AringJosh PNo ratings yet
- MeasurementDocument7 pagesMeasurementJosh PNo ratings yet
- The Periodic Table Unit: Honors ExtensionsDocument6 pagesThe Periodic Table Unit: Honors ExtensionsJosh PNo ratings yet
- Activity 8: Metal Identification Metal Activity Series: Prep AringDocument6 pagesActivity 8: Metal Identification Metal Activity Series: Prep AringJosh PNo ratings yet
- Density Practice Problems: For The Problems Below, Show All Work and Box Your Final AnswersDocument2 pagesDensity Practice Problems: For The Problems Below, Show All Work and Box Your Final AnswersJosh PNo ratings yet
- Ac2 Forensics GlassdensitiesDocument4 pagesAc2 Forensics GlassdensitiesJosh PNo ratings yet
- Ac7 IEgraphDocument1 pageAc7 IEgraphJosh PNo ratings yet
- Ac7 ForensicsDocument2 pagesAc7 ForensicsJosh PNo ratings yet
- Ac5 Forensics WhitepowdersDocument9 pagesAc5 Forensics WhitepowdersJosh PNo ratings yet
- AbsgraphsDocument1 pageAbsgraphsJosh PNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Weymouth and Panhandle Equations For Gas PipelinesDocument4 pagesWeymouth and Panhandle Equations For Gas PipelinessgrsthNo ratings yet
- Kepes Gyorgy 1967 Light and DesignDocument31 pagesKepes Gyorgy 1967 Light and DesignAnonymous TOMo4s100% (1)
- Psabits WithanswersDocument12 pagesPsabits WithanswersChinnareddy KarriNo ratings yet
- Chapt 6Document15 pagesChapt 6morphos777No ratings yet
- Production Engineering Reference Books For GATEDocument3 pagesProduction Engineering Reference Books For GATEkavi_soniiNo ratings yet
- Total StationDocument7 pagesTotal Stationfaizankhan23No ratings yet
- Meshing in ANSYS WorkbenchDocument5 pagesMeshing in ANSYS Workbenchfr129834No ratings yet
- ANU MATH2405 Notes - 1Document40 pagesANU MATH2405 Notes - 1James KingNo ratings yet
- Introduction BearingDocument4 pagesIntroduction BearingtonojsgpNo ratings yet
- Magnetic Induction and Electric Potential PDFDocument60 pagesMagnetic Induction and Electric Potential PDFDavid Garrido GonzalezNo ratings yet
- Cartography Unit 3: Basic Geodesy Flashcards - QuizletDocument4 pagesCartography Unit 3: Basic Geodesy Flashcards - QuizletTJ CabatinganNo ratings yet
- Chemistry 311:: Instructor: Course DescriptionDocument33 pagesChemistry 311:: Instructor: Course DescriptionPaul Venson RaraNo ratings yet
- Report On Dimensional Tolerance: DefinationDocument10 pagesReport On Dimensional Tolerance: DefinationPiyush BariNo ratings yet
- 4.1 Gravity Force LabDocument4 pages4.1 Gravity Force LabT. Danielle Dockery100% (1)
- Determination of Coefficient of Permeability Using Constant-Head Test and Falling Head TestDocument7 pagesDetermination of Coefficient of Permeability Using Constant-Head Test and Falling Head TestVerra Myza AratNo ratings yet
- Hysteresis Modelling For A MR Damper: Rmm@itesm - MX (Ruben Morales-Menendez)Document9 pagesHysteresis Modelling For A MR Damper: Rmm@itesm - MX (Ruben Morales-Menendez)dvarsastryNo ratings yet
- FluorosensDocument14 pagesFluorosensabdul wahidNo ratings yet
- Sika Membrane 2000Document6 pagesSika Membrane 2000Anonymous 2Dz4Kq9M7No ratings yet
- Which Is Formed First, in Structural Geology Fold Then Fault or Fault Then FoldDocument13 pagesWhich Is Formed First, in Structural Geology Fold Then Fault or Fault Then Foldanicetus namangNo ratings yet
- IAL Physics Jan 2017 Unit 4 QP PDFDocument24 pagesIAL Physics Jan 2017 Unit 4 QP PDFsabreenNo ratings yet
- CP S HW CH 5 DetailedDocument9 pagesCP S HW CH 5 Detailedanon_156453388No ratings yet
- Scientific Attitudes and ValuesDocument12 pagesScientific Attitudes and ValuesSadik Khan83% (6)
- Fe Environmental EngineeringDocument24 pagesFe Environmental Engineeringvzimak2355No ratings yet
- Kinetic Study of A Gas - Phase Catalytic Packed Bed Membrane ReactorDocument33 pagesKinetic Study of A Gas - Phase Catalytic Packed Bed Membrane ReactorSukaran SinghNo ratings yet
- Aguilar Et Al, 2000Document8 pagesAguilar Et Al, 2000Edgar SilveiraNo ratings yet
- Glassomelt - Indian CalumiteDocument5 pagesGlassomelt - Indian CalumiteSourabh JainNo ratings yet
- CFX MultiphaseDocument2 pagesCFX MultiphaseNiamul Baqui100% (1)
- Chem 11 Mole Review Ch3 PDFDocument4 pagesChem 11 Mole Review Ch3 PDFCarl Agape DavisNo ratings yet
- Methods For Assessing The Stability of Slopes During Earthquakes-A RetrospectiveDocument20 pagesMethods For Assessing The Stability of Slopes During Earthquakes-A Retrospectiveilijarsk100% (1)
- Turbulence Openfoam PDFDocument31 pagesTurbulence Openfoam PDFMidhun MvNo ratings yet