Professional Documents
Culture Documents
Acid-Base Titration Lab
Uploaded by
Elizabeth Phillips0 ratings0% found this document useful (0 votes)
1K views1 pageAcid-base Titration is the measured amount of a solution of unknown concentration is added to a known volume of a second solution until the reaction between them has reached equivalence point. We titrated together KHP and NaOH. An acid is a compound that we combined with another substance and loses a hydrogen ion.
Original Description:
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentAcid-base Titration is the measured amount of a solution of unknown concentration is added to a known volume of a second solution until the reaction between them has reached equivalence point. We titrated together KHP and NaOH. An acid is a compound that we combined with another substance and loses a hydrogen ion.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
1K views1 pageAcid-Base Titration Lab
Uploaded by
Elizabeth PhillipsAcid-base Titration is the measured amount of a solution of unknown concentration is added to a known volume of a second solution until the reaction between them has reached equivalence point. We titrated together KHP and NaOH. An acid is a compound that we combined with another substance and loses a hydrogen ion.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 1
Acid-Base Titration (Determination of Ka and Pka)
2/2/11
Purpose: To calculate the Ka and pKa of potassium hydrogen
phthalate.
Procedure: Measure about 0.4 grams of KHP into water and using a
buret measure out 1mL at a time of NaOH and graph the pH.
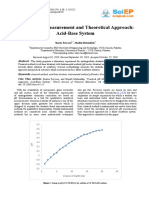
Calculations: See graph for calculations.
Conclusion: We came to the conclusion that the equivalence point is
about a pH of 9 and the half way point is about 5.6 giving us a percent
error of 3.7%.
Discussion of Theory: The equivalence point, which is when the acid
and base are stoichiometrically equilvalent. . The equivalence
point in the lab was found to be about a pH of 9. Titration is the
measured amount of a solution of unknown concentration is
added to a known volume of a second solution until the reaction
between them has reached equivalence point. We titrated
together KHP and NaOH. An acid is a compound that we
combined with another substance and loses a hydrogen ion. A
base is a compound that gains a hydrogen ion when combined
with another substance
Sources of Error: A problem we could have is when we are stirring the
solution with a magnetic rod the solution could splash out giving
us a larger percent error.
Sources:
www.chemguide.co.uk/physical/acidbaseeqia/phcurves.html
http://wordnetweb.princeton.edu/perl/webwn?s=acid
http://wordnetweb.princeton.edu/perl/webwn?s=base
You might also like
- Sample Lab Report For Experiment 2Document2 pagesSample Lab Report For Experiment 2Ashfaq AhmadNo ratings yet
- IP 4. Protocol - Chemical Principles II LaboratoryDocument9 pagesIP 4. Protocol - Chemical Principles II LaboratoryJavier PratdesabaNo ratings yet
- Post Lab 5Document7 pagesPost Lab 5Heinrich SolivenNo ratings yet
- 6 - IonizationDocument48 pages6 - IonizationYashfa YasinNo ratings yet
- IP 3. Protocol - Chemical Principles II LaboratoryDocument9 pagesIP 3. Protocol - Chemical Principles II LaboratoryJavier PratdesabaNo ratings yet
- PH and BufferDocument3 pagesPH and BufferMuhammad YaseenNo ratings yet
- Titration Lab ReportDocument38 pagesTitration Lab Reportadillaanis100% (4)
- Identifying An Unknown Weak Acids ExperimentDocument18 pagesIdentifying An Unknown Weak Acids Experimentgeek3112100% (5)
- BI309 Lab 3 Ryan CarrollDocument4 pagesBI309 Lab 3 Ryan CarrollRyan CarrollNo ratings yet
- Berea gl05 LabDocument7 pagesBerea gl05 LabGregorio Antonio Valero VerdeNo ratings yet
- General Chemistry 2 Quarter 4 - Week 4 Module 4: PH of Buffer SolutionsDocument12 pagesGeneral Chemistry 2 Quarter 4 - Week 4 Module 4: PH of Buffer SolutionsHazel EncarnacionNo ratings yet
- Experiment Number 7Document6 pagesExperiment Number 7api-457258209No ratings yet
- Completing Lab Data: Acids Bases and BuffersDocument7 pagesCompleting Lab Data: Acids Bases and Buffers蔺雄人No ratings yet
- Expt.1 BiochemDocument4 pagesExpt.1 BiochemMc de RamosNo ratings yet
- Bclab FR 1Document4 pagesBclab FR 1Natalie CuNo ratings yet
- Introduction BufferDocument2 pagesIntroduction Bufferxuni34No ratings yet
- Exp 2Document24 pagesExp 2Dhiyyah MardhiyyahNo ratings yet
- pH Titration Lab Experiment Ka DeterminationDocument4 pagespH Titration Lab Experiment Ka DeterminationxmusiqaNo ratings yet
- Half Titration Lab ReportDocument6 pagesHalf Titration Lab Reportapi-20078641867% (3)
- Acid-Base Titrations Curve Formal LabDocument9 pagesAcid-Base Titrations Curve Formal LabAshley StraubNo ratings yet
- Ka & Molar Mass of a Weak AcidDocument7 pagesKa & Molar Mass of a Weak AcidLeslie Sarah100% (1)
- AP Chemistry Lab Brockport High School NY USA Titration of Acids and Bases MR KeeferDocument2 pagesAP Chemistry Lab Brockport High School NY USA Titration of Acids and Bases MR KeeferMuhammad Arif LangaahNo ratings yet
- 5: PH Measurement and Its Applications (Experiment) : ObjectivesDocument19 pages5: PH Measurement and Its Applications (Experiment) : ObjectivesNajmi NasirNo ratings yet
- Chemistry Lab Report1Document22 pagesChemistry Lab Report1RoseAnne BellaNo ratings yet
- Experiment 10 - Quantitative Determination of The Purity and Dissociation of Potassium Hydrogen Phthalate by Potentiometric Titration AtqDocument3 pagesExperiment 10 - Quantitative Determination of The Purity and Dissociation of Potassium Hydrogen Phthalate by Potentiometric Titration AtqDoom RefugeNo ratings yet
- 2.0 Literature Review 2.1 PH: PH Log pOH Log PH 14 pOHDocument13 pages2.0 Literature Review 2.1 PH: PH Log pOH Log PH 14 pOHNorzulaika AmitNo ratings yet
- Chem 18.1 Titration AnalysisDocument5 pagesChem 18.1 Titration AnalysisNicole NatanauanNo ratings yet
- Sample Chemistry Undergraduate Laboratory ReportDocument14 pagesSample Chemistry Undergraduate Laboratory ReportApril TapayanNo ratings yet
- GARCIA LabNotebook 4111LDocument5 pagesGARCIA LabNotebook 4111LKrizzi Dizon GarciaNo ratings yet
- GARCIA LabNotebook 4111LDocument5 pagesGARCIA LabNotebook 4111LKrizzi Dizon GarciaNo ratings yet
- Exp 2 Determination of The Ka ValueDocument21 pagesExp 2 Determination of The Ka ValueSYahira HAzwaniNo ratings yet
- Maintaining Constant pH: Blood Buffer System and Factors Affecting Buffer CapacityDocument31 pagesMaintaining Constant pH: Blood Buffer System and Factors Affecting Buffer CapacityJoyce Castil (Joyceee)No ratings yet
- Biological Buffer SystemDocument6 pagesBiological Buffer SystemJason Raquin RoqueNo ratings yet
- Lab 2 Eng Chem LabDocument19 pagesLab 2 Eng Chem LabillyzlNo ratings yet
- Determining Vinegar Acidity Through TitrationDocument15 pagesDetermining Vinegar Acidity Through TitrationDayledaniel SorvetoNo ratings yet
- Potentiometric Determination of Phosphoric Acid in Unknown SampleDocument7 pagesPotentiometric Determination of Phosphoric Acid in Unknown SamplekahullanyNo ratings yet
- Potentiometric determination of phosphoric acid contentDocument7 pagesPotentiometric determination of phosphoric acid contentFlex GodNo ratings yet
- Lab 9 2020 2Document22 pagesLab 9 2020 2Kushalpratap SinghNo ratings yet
- 1Document8 pages1Isma WantiNo ratings yet
- PDF PH TITRATIONDocument16 pagesPDF PH TITRATIONعبدالله هنيةNo ratings yet
- CPB 30103 Biochemical Engineering UniKL MICET Experiment 1: Preparation of Buffer Solution Full Lab ReportDocument10 pagesCPB 30103 Biochemical Engineering UniKL MICET Experiment 1: Preparation of Buffer Solution Full Lab ReportSiti Hajar Mohamed0% (1)
- CH142Exp5Titration PDFDocument7 pagesCH142Exp5Titration PDFSako RasheedNo ratings yet
- pH Measurement and Buffer PreparationDocument7 pagespH Measurement and Buffer PreparationPatricia Camryne AmbidaNo ratings yet
- Johanna Protheroe Titration PaperDocument10 pagesJohanna Protheroe Titration PaperLoves to SkiNo ratings yet
- pH Titration of Unknown Acid MixtureDocument18 pagespH Titration of Unknown Acid MixturemesasysillasNo ratings yet
- Lab Titration of VinegarDocument5 pagesLab Titration of Vinegardesree07No ratings yet
- WjceDocument5 pagesWjceshmohiuddinNo ratings yet
- Lab Report 1Document5 pagesLab Report 1cuttlefishobat100% (5)
- Determination of pKa for Weak AcidDocument5 pagesDetermination of pKa for Weak AcidSonu DubeyNo ratings yet
- Determination Acetic AcidDocument21 pagesDetermination Acetic Acidameyakem100% (1)
- Experiment 1: Measuring The PH Value of WaterDocument5 pagesExperiment 1: Measuring The PH Value of Waternur aujiNo ratings yet
- Determining Acid Concentration Through TitrationDocument8 pagesDetermining Acid Concentration Through TitrationPamNo ratings yet
- Acid Base Titration Lab 6Document11 pagesAcid Base Titration Lab 6Jose Cencič0% (1)
- PH and Buffer Measurement Formal Report PDFDocument4 pagesPH and Buffer Measurement Formal Report PDFGLENN TANNo ratings yet
- Experiment 7Document4 pagesExperiment 7Vinitra PillaiNo ratings yet
- AP Chemistry Investigation 4 - Judy, Paul, AnthonyDocument13 pagesAP Chemistry Investigation 4 - Judy, Paul, AnthonyAnthony HowerNo ratings yet
- Experiment 1 Preparation of Buffer SolutionsDocument16 pagesExperiment 1 Preparation of Buffer Solutionsmohamad ashaziq89% (56)
- Titration Lab ReportDocument25 pagesTitration Lab ReportVarit SuriyasomboonNo ratings yet
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet