Professional Documents
Culture Documents
ALT
Uploaded by
Liviu Athos TamasOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ALT
Uploaded by
Liviu Athos TamasCopyright:
Available Formats
INSTRUCTIONS FOR USE ALT
VITROS Chemistry Products ALT Slides Alanine
Aminotransferase
153 7513
165 5281
Intended Use
For in vitro diagnostic use only.
VITROS ALT Slides quantitatively measure alanine aminotransferase (ALT) activity in serum and plasma.
Summary and Explanation of the Test
Alanine aminotransferase is present in high activity in liver, skeletal muscle, heart, and kidney. Serum ALT increases rapidly in
liver cell necrosis, hepatitis, hepatic cirrhosis, liver tumors, obstructive jaundice, Reye’s syndrome, extensive trauma to skeletal
muscle, myositis, myocarditis, and myocardial infarction. 1
Principles of the Procedure
The VITROS ALT Slide method is performed using the VITROS ALT Slides and the VITROS Chemistry Products Calibrator
Kit 3 on VITROS Chemistry Systems.
The VITROS ALT Slide is a multilayered, analytical element coated on a polyester support.
A drop of patient sample is deposited on the slide and is evenly distributed by the spreading layer to the underlying layers. The
spreading layer contains the ALT substrates L-alanine and sodium α-ketoglutarate. Alanine aminotransferase catalyzes the
transfer of the amino group of L-alanine to α-ketoglutarate to produce pyruvate and glutamate. Lactate dehydrogenase (LDH)
then catalyzes the conversion of pyruvate and NADH to lactate and NAD+.
The rate of oxidation of NADH is monitored by reflectance spectrophotometry. The rate of change in reflection density is
proportional to enzyme activity.
Reaction Sequence
ALT
alanine + α-ketoglutarate pyruvate + glutamate
pyridoxal-5-phosphate
+ LDH +
pyruvate + NADH + H lactate + NAD
Test Type and Conditions
Test Type and Conditions for ALT
Approximate Sample Drop
Test Type VITROS System Incubation Time Temperature Wavelength Volume
Multiple-point rate 5,1 FS, 950, 750, 550, 250 5 minutes 37°C (98.6°F) 340 nm 11 µL
Warnings and Precautions
For in vitro diagnostic use only.
Take care when handling materials and samples of human origin. Since no test method can offer complete assurance that
infectious agents are absent, consider all clinical specimens, controls, and calibrators potentially infectious. Handle specimens,
solid and liquid waste, and test components in accordance with local regulations and NCCLS Guideline M29 2 or other
published biohazard safety guidelines.
For specific warnings and precautions for calibrators, quality control materials, and other components, refer to the Instructions
for Use for the appropriate VITROS product, or to other manufacturer’s product literature.
Version 3.0 Pub. No. MP2-36_EN 1
ALT INSTRUCTIONS FOR USE
Alanine Aminotransferase Reagents
Reagents
Slide Diagram
Slide Ingredients 1. Upper slide mount
2 2. Spreading layer (BaSO4)
Reactive ingredients per cm • sodium α–ketoglutarate
• L-alanine
Lactate dehydrogenase (porcine muscle, E.C.1.1.1.27) 0.12 U; 3. Reagent layer
L-alanine 0.86 mg; sodium α-ketoglutarate 54 µg; nicotinamide adenine • buffer, pH 8.0
dinucleotide, reduced 35 µg; and sodium pyridoxal-5-phosphate 11 µg. • lactate dehydrogenase
• NADH
Other ingredients • pyridoxal-5-phosphate
4. Support layer
Pigment, binders, buffer, surfactants, cross-linking agent and stabilizer. 5. Lower slide mount
Cartridge Handling
CAUTION: Do not use slide cartridges with damaged or incompletely sealed packaging.
• Inspect the packaging for signs of damage.
• Be careful when opening the outer packaging with a sharp instrument so as to avoid damage to the individual product
packaging.
Cartridge Preparation
IMPORTANT: The slide cartridge must reach room temperature, 18°–28°C (64°–82°F), before it is
unwrapped and loaded into the slide supply.
1. Remove the slide cartridges from storage.
2. Warm the wrapped cartridge at room temperature for 30 minutes when taken from the refrigerator or 60 minutes from the
freezer.
3. Unwrap and load the cartridge into the slide supply.
NOTE: Load the cartridges within 24 hours after they reach room temperature,
18°–28°C (64°–82°F).
Slide Storage and Stability
VITROS ALT Slides are stable until the expiration date on the carton when they are stored and handled as specified.
Slide Storage and Stability for ALT
Slide Cartridges Storage Condition Stability
Unopened Refrigerated 2°–8°C (36°–46°F) Until expiration date
Frozen ≤-18°C (≤0°F) Until expiration date
Opened On-analyzer System turned on ≤4 weeks
On-analyzer System turned off ≤2 hours
• Verify performance with quality control materials:
– If the system is turned off for more than 2 hours.
– After reloading cartridges that have been removed from the slide supply and stored for later use.
Specimen Requirements
WARNING: Handle specimens as biohazardous material.
Specimens Recommended
• Serum
• Plasma: EDTA
Heparin
IMPORTANT: Certain collection devices have been reported to affect other analytes and tests. 3
Confirm that your collection devices are compatible with this test.
Specimens Not Recommended
• Do not use hemolyzed specimens. 4
2 Pub. No. MP2-36_EN Version 3.0
INSTRUCTIONS FOR USE ALT
Testing Procedure Alanine Aminotransferase
Serum and Plasma
Specimen Collection and Preparation
Collect specimens using standard laboratory procedures. 5, 6
NOTE: For details on minimum fill volume requirements, refer to the operating instructions for
your VITROS Chemistry System.
Patient Preparation
• No special patient preparation is necessary.
Special Precautions
• Centrifuge specimens and remove the serum or plasma from the cellular material within 3 days of collection. 7
Specimen Handling and Storage
WARNING: Handle specimens as biohazardous material.
• Handle and store specimens in stoppered containers to avoid contamination and evaporation.
• Mix samples by gentle inversion and bring to room temperature, 18°–28°C (64°–82°F), prior to analysis.
IMPORTANT: Do not freeze the specimen.
Specimen Storage and Stability for ALT: Serum and Plasma 7
Storage Temperature Stability
Room temperature 18°–28°C (64°–82°F) ≤3 days
Refrigerated 2°–8°C (36°–46°F) ≤1 week
Frozen ≤-18°C (≤0°F) Not recommended
Testing Procedure
Materials Provided
• VITROS Chemistry Products ALT Slides
Materials Required But Not Provided
• VITROS Chemistry Products Calibrator Kit 3
• Quality control materials, such as VITROS Chemistry Products Performance Verifier I and II
• VITROS Chemistry Products 7% BSA
• VITROS Chemistry Products FS Diluent Pack 2 (BSA/Saline) (for on-analyzer dilution)
Operating Instructions
• Check reagent inventories at least daily to ensure that quantities are sufficient for the planned workload.
• For additional information, refer to the operating instructions for your VITROS Chemistry System.
IMPORTANT: Bring all fluids and samples to room temperature, 18°–28°C (64°–82°F), prior to
analysis.
Sample Dilution
Serum and Plasma
If alanine aminotransferase activities exceed the system’s reportable (dynamic) range:
Manual Sample Dilution
1. Dilute the sample with VITROS 7% BSA.
2. Reanalyze.
3. Multiply the results by the dilution factor to obtain an estimate of the original sample’s alanine aminotransferase activity.
On-Analyzer Sample Dilution (VITROS 5,1 FS and VITROS 250 only)
Refer to the VITROS Chemistry System operating instructions for more information on the On-Analyzer Dilution Procedure. For
VITROS 5,1 FS, use VITROS Chemistry Products FS Diluent Pack 2 for the dilution.
Version 3.0 Pub. No. MP2-36_EN 3
ALT INSTRUCTIONS FOR USE
Alanine Aminotransferase Calibration
Calibration
Required Calibrators
VITROS Chemistry Products Calibrator Kit 3
Calibrator Preparation, Handling, and Storage
Refer to the Instructions for Use for VITROS Calibrator Kit 3.
Calibration Procedure
Refer to the operating instructions for your VITROS Chemistry System.
When to Calibrate
Calibrate:
• When the slide lot number changes.
• When critical system parts are replaced due to service or maintenance.
• When government regulations require.
– For example, in the USA, CLIA regulations require calibration or calibration verification at least once every six months.
The VITROS ALT test may also need to be calibrated:
• If quality control results are consistently outside acceptable range.
• After certain service procedures have been performed.
For additional information, refer to the operating instructions for your VITROS Chemistry System.
Calculations
Based on sequential readings of the slide’s reflectance at 340 nm over the defined incubation period, a rate of change in
reflectance is determined. This rate is used in the software-resident multi-point rate calibration model to compute enzyme
activity. Once a calibration has been performed for each slide lot, alanine aminotransferase activity in unknown samples can be
determined from the rate of change in reflectance measured for each unknown test slide.
Validity of a Calibration
Calibration parameters are automatically assessed by the VITROS Chemistry System against a set of quality parameters
detailed in the Coefficients and Limits screen (for VITROS 5,1 FS, see the Review Assay Data screen). Failure to meet any of
the pre-defined quality parameters results in a failed calibration. The calibration report should be used in conjunction with
quality control results to determine the validity of a calibration.
Reportable (Dynamic) Range
Reportable (Dynamic) Range for ALT
Conventional and SI Units Alternate Units
(U/L) (µkat/L)
3–1000 0.05–16.70
For out-of-range samples, refer to “Sample Dilution.”
Traceability of the Calibration
Values assigned to the VITROS Chemistry Products Calibrator Kit 3 for alanine aminotransferase are traceable to the alanine
aminotransferase method recommended by the International Federation of Clinical Chemistry (IFCC), 8 adapted to a centrifugal
analyzer at 37°C.
Quality Control
Procedure Recommendations
WARNING: Handle quality control materials as biohazardous material.
• Choose control levels that check the clinically relevant range.
• Analyze quality control materials in the same manner as patient samples, before or during patient sample processing.
• To verify system performance, analyze control materials:
– After calibration.
– According to local regulations or at least once each day that the test is being performed.
– After specified service procedures are performed. Refer to the operating instructions for your VITROS Chemistry
System.
• If control results fall outside your acceptable range, investigate the cause before deciding whether to report patient results.
4 Pub. No. MP2-36_EN Version 3.0
INSTRUCTIONS FOR USE ALT
Expected Values and Reporting Units Alanine Aminotransferase
• For general quality control recommendations, refer to Statistical Quality Control for Quantitative Measurements: Principles
and Definitions; Approved Guideline-Second Edition 9 or other published guidelines.
• For additional information, refer to the operating instructions for your VITROS Chemistry System.
Quality Control Material Selection
IMPORTANT: VITROS Performance Verifiers are recommended for use with the VITROS Chemistry
System. Evaluate the performance of other commercial control fluids for compatibility
with this test before using for quality control.
• Control materials other than VITROS Performance Verifiers may show a difference when compared with other alanine
aminotransferase methods if they:
– Depart from a true human matrix.
– Contain high concentrations of preservatives, stabilizers, or other nonphysiological additives.
• Enzyme activity might also vary with enzyme source, diluent temperature, and activation time during reconstitution.
• Do not use control materials stabilized with ethylene glycol.
Quality Control Material Preparation and Storage
Refer to the Instructions for Use for VITROS Chemistry Products Performance Verifier I and II or to other manufacturer's
product literature.
Expected Values and Reporting Units
Reference Interval
These reference intervals are the central 95% of results from an internal study of 2444 apparently healthy adults (547 females
and 1897 males).
Reference Interval for ALT
Conventional and SI Units Alternate Units
(U/L) (µkat/L)
Adult 13–69 0.2–1.2
Females 9–52 0.2–0.9
Males 21–72 0.4–1.2
Each laboratory should confirm the validity of these intervals for the population it serves.
Reporting Units and Unit Conversion
The VITROS Chemistry System may be programmed to report ALT results in conventional, SI, and alternate units.
Reporting Units and Unit Conversion for ALT
Conventional/SI Units Alt Units
U/L µkat/L (U/L x 0.0167)
Limitations of the Procedure
Known Interferences
None identified.
Other Limitations
Certain drugs and clinical conditions are known to alter alanine aminotransferase activity in vivo. For additional information,
refer to one of the published summaries. 10, 11
Version 3.0 Pub. No. MP2-36_EN 5
ALT INSTRUCTIONS FOR USE
Alanine Aminotransferase Performance Characteristics
Performance Characteristics
Method Comparison
The plot and table show the results of a comparison of samples analyzed on the VITROS 750 System with those analyzed
using the IFCC comparative method, 8 adapted to a centrifugal analyzer at 37°C. Testing followed NCCLS Protocol EP9. 12
The table also shows the results of comparisons of the VITROS 250 and 950 Systems with the VITROS 750 System, and
comparisons of the 5,1 FS System with the 950 System.
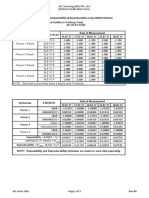
Method Comparison for ALT: Serum
Conventional and SI Units Alternate Units
VITROS 750 System (µkat/L)
VITROS 750 System (U/L)
Comparative Method: Modified IFCC at 37°C Comparative Method: Modified IFCC at 37°C
(U/L) (µkat/L)
Method Comparison for ALT: Serum
Conventional and SI Units (U/L) Alternate Units (µkat/L)
Correlation Range of Range of
n Slope Coefficient Sample Activity Intercept Sy.x Sample Activity Intercept Sy.x
750 System vs.
195 1.02 0.997 4–911 +9.2 20.3 0.1–15.2 +0.15 0.34
comparative method
250 System vs.
60 1.01 0.999 14–373 -0.6 2.6 0.2–6.2 -0.01 0.04
750 System
950 System vs.
122 0.99 0.999 9–988 -0.9 2.4 0.2–16.5 -0.02 0.04
750 System
5,1 FS System vs.
134 1.00 1.000 11–886 +1.7 2.3 0.2–14.8 +0.03 0.04
950 System
Precision
Precision was evaluated with quality control materials on VITROS 250, 750, 950, and 5,1 FS Systems following NCCLS
Protocol EP5. 13
The data presented are a representation of test performance and are provided as a guideline. Variables such as sample
handling and storage, reagent handling and storage, laboratory environment, and system maintenance can affect
reproducibility of test results.
6 Pub. No. MP2-36_EN Version 3.0
INSTRUCTIONS FOR USE ALT
References Alanine Aminotransferase
Precision for ALT: Serum
Conventional and SI Units (U/L) Alternate Units (µkat/L) Within
Mean Within Within Mean Within Within Lab No. No.
System Activity Day SD* Lab SD** Activity Day SD* Lab SD** CV%** Observ. Days
VITROS 250 37 3.5 4.1 0.6 0.06 0.07 11.2 78 20
204 3.8 4.9 3.4 0.06 0.08 2.4 78 20
VITROS 750 34 2.0 2.7 0.6 0.03 0.05 8.1 90 23
189 2.3 3.7 3.2 0.04 0.06 2.0 92 23
VITROS 950 34 1.7 3.2 0.6 0.03 0.05 9.6 84 23
186 1.7 3.2 3.1 0.03 0.05 1.7 86 23
VITROS 5,1 FS 44 1.6 2.9 0.7 0.03 0.05 6.6 96 22
187 1.9 3.5 3.1 0.03 0.06 1.9 96 22
* Within Day precision was determined using two runs/day with two to three replications.
** Within Lab precision was determined using a single lot of slides and calibrating weekly.
Specificity
Substances That Do Not Interfere
The substances listed in the table were tested with VITROS ALT Slides following NCCLS Protocol EP7 14 and found not to
interfere, bias <5.5 U/L, at the concentration shown.
Substances That Do Not Interfere With ALT
Compound Concentration Compound Concentration
AST 200 U/L 200 U/L Glutathione 1 mg/dL 33 µmol/L
Ascorbic Acid 3 mg/dL 170 µmol/L Intralipid 800 mg/dL 8 g/L
Bilirubin 40 mg/dL 684 µmol/L Salicylic acid 35 mg/dL 2.5 mmol/L
Ethanol 300 mg/dL 65 mmol/L
References
1. Tietz NW (ed). Fundamentals of Clinical Chemistry. ed. 3. Philadelphia: WB Saunders; 369–371; 1987.
2. NCCLS. Protection of Laboratory Workers from Instrument Biohazards and Infectious Diseases Transmitted by Blood, Body Fluids and
Tissue; Approved Guideline. NCCLS Document M29 (ISBN 1-56238). NCCLS, Wayne, PA 19087; 1997.
3. Calam RR. Specimen Processing Separator Gels: An Update. J Clin Immunoassay. 11:86–90; 1988.
4. Young DS. Effects of Preanalytical Variables on Clinical Laboratory Tests. Washington D.C.: AACC Press; 3–7; 1993.
5. NCCLS. Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture. NCCLS Document H3. Wayne, PA: NCCLS;
1991.
6. NCCLS. Procedures for the Collection of Diagnostic Blood Specimens by Skin Puncture. NCCLS Document H4. Wayne, PA: NCCLS;
1991.
7. Clinical Laboratory Handbook for Patient Preparation and Specimen Handling. Fascicle VI: Chemistry/Clinical Microscopy. Northfield,
IL: College of American Pathologists; 1992.
8. Bergmeyer HU, Horder M, Rej R. Approved Recommendation (1985) on IFCC Methods for the Measurement of Catalytic Concentration
of Enzymes. Part 3. IFCC Method for Alanine Aminotransferase. J. Clin. Chem. Clin. Biochem. 24:481; 1986.
9. NCCLS. Statistical Quality Control for Quantitative Measurements: Principles and Definitions; Approved Guideline-Second Edition.
NCCLS Document C24. Wayne, PA: NCCLS; 1999.
10. Young DS. Effects of Drugs on Clinical Laboratory Tests. ed. 4. Washington D.C.: AACC Press; 1995.
11. Friedman RB, Young DS. Effects of Disease on Clinical Laboratory Tests. Washington, D.C.: AACC Press; 1990.
12. NCCLS. Method Comparison and Bias Estimation Using Patient Samples; Approved Guideline. NCCLS Document EP9. Wayne, PA:
NCCLS; 1995.
13. NCCLS. User Evaluation of Precision Performance with Clinical Chemistry Devices. NCCLS Document EP5. Wayne, PA: NCCLS;
1992.
14. NCCLS. Interference Testing in Clinical Chemistry. NCCLS Document EP7. Wayne, PA: NCCLS; 1986.
Version 3.0 Pub. No. MP2-36_EN 7
ALT INSTRUCTIONS FOR USE
Alanine Aminotransferase Glossary of Symbols
Glossary of Symbols
8 Pub. No. MP2-36_EN Version 3.0
INSTRUCTIONS FOR USE ALT
Revision History Alanine Aminotransferase
Revision History
Date of
Revision Version Description of Technical Changes*
2004-09-13 3.0 • Added VITROS 5,1 FS Chemistry System
• Specificity – added Intralipid; updated Bilirubin
• Glossary of Symbols – updated data
2003-07-28 2.0 • New organization and sections consistent with IVD Directive
• Reference Interval – adult: corrected values
• Limitations of the Procedure – removed statements regarding elevated total
protein
• Method Comparison – updated comparisons for 750 System and plots
• Precision – updated data for the 750 System
• References – added 2, 3, 12, 13, 14
2002APR19 1.0 – English only New format, technically equivalent to 11/96.
* The change bars indicate the position of a technical amendment to the text with respect to the previous version of the document.
When this Instructions For Use is replaced, sign and date below and retain as specified by local regulations or laboratory
policies, as appropriate.
_________________________________ _____________
Signature Obsolete Date
Version 3.0 Pub. No. MP2-36_EN 9
ALT INSTRUCTIONS FOR USE
Alanine Aminotransferase
Ortho-Clinical Diagnostics Brasil:
Johnson & Johnson Distribuidor:Johnson&Johnson Produtos Profissionais LTDA
50-100 Holmers Farm Way Rod. Presidente Dutra, Km 154, S.J. dos Campos – SP –
High Wycombe CEP: 12240-908 - Brasil
Buckinghamshire CNPJ:54.516.661/0002-84
HP12 4DP Farm.Resp.: Nancy M.R.B Lopes C.R.F.-SP N° 10965
United Kingdom SAC : 0800787865
Ortho-Clinical Diagnostics, Inc. VITROS is a trademark of Ortho-Clinical Diagnostics, Inc.
100 Indigo Creek Drive © Ortho-Clinical Diagnostics, Inc., 2003, 2004.
Rochester, NY 14626-5101
10 Pub. No. MP2-36_EN Version 3.0
You might also like
- Sans 10137 2011Document107 pagesSans 10137 2011Pamps Mangampo50% (2)
- Lab Policies Hemoglobin A1C - Cobas c501 Lab 4004Document6 pagesLab Policies Hemoglobin A1C - Cobas c501 Lab 4004yosefinNo ratings yet
- eCL8000 Installation GuideDocument87 pageseCL8000 Installation Guidegerente soportecNo ratings yet
- Lyphochek Immunoassay Plus Control Levels 1, 2 and 3Document26 pagesLyphochek Immunoassay Plus Control Levels 1, 2 and 3Aniket dubeyNo ratings yet
- Concrete CanvasDocument20 pagesConcrete CanvasJet Espejon JavierNo ratings yet
- Totalt4 ArcDocument6 pagesTotalt4 Arctesteste testeNo ratings yet
- CREJ2Document4 pagesCREJ2ARIF AHAMMED PNo ratings yet
- TotalBhCG ARCDocument7 pagesTotalBhCG ARCLau GómezNo ratings yet
- Insert - Elecsys Syphilis - Ms 07802960190.V3.EnDocument5 pagesInsert - Elecsys Syphilis - Ms 07802960190.V3.EnGuneyden GuneydenNo ratings yet
- Cholesterol Specimen Collection & Handling GuideDocument4 pagesCholesterol Specimen Collection & Handling GuideCriiiiisl100% (1)
- T4 IflashDocument4 pagesT4 IflashNIGHT tubeNo ratings yet
- Totalt3 ArcDocument6 pagesTotalt3 ArcTanveerNo ratings yet
- Rheumatoid Factors: InstrumentsDocument1 pageRheumatoid Factors: InstrumentsEnrique DuarteNo ratings yet
- Elecsys BRAHMS PCT: ProcalcitoninDocument5 pagesElecsys BRAHMS PCT: ProcalcitoninMilagrosLcNo ratings yet
- Sysmex XW - 100: Instructions For Use ManualDocument32 pagesSysmex XW - 100: Instructions For Use ManualNahom BalchaNo ratings yet
- CL - 1000i Immunofluorescence Analyzer.Document6 pagesCL - 1000i Immunofluorescence Analyzer.bikouvoNo ratings yet
- BS-200 Brochura ENDocument3 pagesBS-200 Brochura ENmdkNo ratings yet
- Budi Altgpt - Doc NewDocument3 pagesBudi Altgpt - Doc NewIrvanda ENVIOUSNo ratings yet
- CYSC2Document4 pagesCYSC2ARIF AHAMMED PNo ratings yet
- ETOHDocument4 pagesETOHARIF AHAMMED PNo ratings yet
- Method Verification of TestDocument2 pagesMethod Verification of TestObak PrithibiNo ratings yet
- ABX Pentra 80 - Service Manual PDFDocument333 pagesABX Pentra 80 - Service Manual PDFFrepa_ALNo ratings yet
- Boeco Autoclave Bte-23D: FeaturesDocument1 pageBoeco Autoclave Bte-23D: FeaturesAdrian Gomez BaldeonNo ratings yet
- Brochure PDFDocument72 pagesBrochure PDFkisa guyNo ratings yet
- 1975ec-2026ec-2028ec 2023-04Document57 pages1975ec-2026ec-2028ec 2023-04Sujit KushwahaNo ratings yet
- Clinical Chemistry Catalogue 2017Document32 pagesClinical Chemistry Catalogue 2017Aleksandra RadonjićNo ratings yet
- Iflash 1800 Kits Quotation For Yhlo ProductsDocument3 pagesIflash 1800 Kits Quotation For Yhlo ProductsMohad Asdel100% (1)
- CA-BA-BS-120 Product IntroductionDocument36 pagesCA-BA-BS-120 Product IntroductionsilentNo ratings yet
- Instructions For Use TSHDocument12 pagesInstructions For Use TSHBenjamin MannNo ratings yet
- G7 Service ManualDocument314 pagesG7 Service Manualzhigang yangNo ratings yet
- Broschyr GEM5000Document12 pagesBroschyr GEM5000Oo Kenx OoNo ratings yet
- H-046-003256-00 TPSA KIT (CLIA) Muti LaguageDocument14 pagesH-046-003256-00 TPSA KIT (CLIA) Muti LaguageSinari AlfatNo ratings yet
- SI Units For Clinical DataDocument6 pagesSI Units For Clinical DataAhmed AliNo ratings yet
- XN-L - Reference Interval From General Information 2017Document4 pagesXN-L - Reference Interval From General Information 2017widiawaty100% (1)
- Control de Calidad 1039Document106 pagesControl de Calidad 1039MARITZA MUÑOZ100% (1)
- 0812 in Vitro Blood Gas Analyzers GuideDocument9 pages0812 in Vitro Blood Gas Analyzers GuidedjebrutNo ratings yet
- Precipath HDL - LDL-C.11818171001.V10.en PDFDocument2 pagesPrecipath HDL - LDL-C.11818171001.V10.en PDFARIF AHAMMED PNo ratings yet
- Ichroma II Test Panels 210331 104829Document2 pagesIchroma II Test Panels 210331 104829Sinergy DiagnosticNo ratings yet
- ACL TOP Family SOP Manual 2016-10-19 enDocument238 pagesACL TOP Family SOP Manual 2016-10-19 enAndra Radulescu100% (1)
- Randox ControlDocument17 pagesRandox ControlLuis Ferdinand Dacera-Gabronino Gamponia-Nonan100% (1)
- BS-380 Service Training: Mindray - A global partnerDocument101 pagesBS-380 Service Training: Mindray - A global partnererrozoNo ratings yet
- Competitive Evaluation of The GEM Premier 3000 With PDFDocument8 pagesCompetitive Evaluation of The GEM Premier 3000 With PDFEllya Latifah IlyasNo ratings yet
- COBAS INTEGRA 400 Plus Analyzer Brochure - Test MenuDocument14 pagesCOBAS INTEGRA 400 Plus Analyzer Brochure - Test Menuvachhanikapil50% (2)
- A1C-2 Whole Blood enDocument6 pagesA1C-2 Whole Blood enSyahdie FahledieNo ratings yet
- FT4 Free Thyroxine (CLIA) Catalog No. Package SizeDocument14 pagesFT4 Free Thyroxine (CLIA) Catalog No. Package SizeSinari AlfatNo ratings yet
- Mindray Bs 200Document13 pagesMindray Bs 200Roberto AriasNo ratings yet
- Catalog (ENG) May Sinh Hoa Tu Dong BS-600Document4 pagesCatalog (ENG) May Sinh Hoa Tu Dong BS-600anhhp8xNo ratings yet
- ECL8000 Installation Guide 220511Document95 pagesECL8000 Installation Guide 220511anhhp8xNo ratings yet
- HItachi 911-912Document41 pagesHItachi 911-912Hồ Thế NguyênNo ratings yet
- Prolactin - IMMULITE and IMMULITE 1000 - Rev 13 DXDCM 09017fe9802977c5-1538194815088Document32 pagesProlactin - IMMULITE and IMMULITE 1000 - Rev 13 DXDCM 09017fe9802977c5-1538194815088Guneyden GuneydenNo ratings yet
- COCIIDocument6 pagesCOCIIARIF AHAMMED PNo ratings yet
- Hemostat Thromboplastin-SI: Determination of Prothrombin Time (PT)Document2 pagesHemostat Thromboplastin-SI: Determination of Prothrombin Time (PT)Lemi MaluluNo ratings yet
- Insert TRIGL 0020767107322COIN V11 enDocument4 pagesInsert TRIGL 0020767107322COIN V11 entechlabNo ratings yet
- Abbott Architect Immunoassay Menu Data SheetDocument4 pagesAbbott Architect Immunoassay Menu Data SheetГалина МиловановаNo ratings yet
- Assay SolutionsDocument1 pageAssay SolutionsAlex RamirezNo ratings yet
- Selectra IDocument18 pagesSelectra IEnrique DuarteNo ratings yet
- Service Manual Humaclot ProDocument8 pagesService Manual Humaclot ProHuy Trung GiápNo ratings yet
- I18f14a en Aia-Cl-1200Document2 pagesI18f14a en Aia-Cl-1200Hary John Tsivery Rakotonjak'sParowNo ratings yet
- Test Menu - MaglumiDocument4 pagesTest Menu - MaglumiLester Freer CascanteNo ratings yet
- H-046-003250-00 CEA KIT (CLIA) Muti LaguageDocument14 pagesH-046-003250-00 CEA KIT (CLIA) Muti LaguageSinari Alfat100% (1)
- c111 Operator Manual PDFDocument502 pagesc111 Operator Manual PDFSuponoNo ratings yet
- Alt TGPDocument8 pagesAlt TGPtesteste testeNo ratings yet
- Applied Biosystems 7300 Real-Time PCR SystemDocument4 pagesApplied Biosystems 7300 Real-Time PCR SystemLiviu Athos TamasNo ratings yet
- Installation and Maintenance Guide: Applied Biosystems 7300/7500/7500 Fast Real-Time PCR SystemDocument178 pagesInstallation and Maintenance Guide: Applied Biosystems 7300/7500/7500 Fast Real-Time PCR SystemLiviu Athos TamasNo ratings yet
- Current Status of The Use of Gene Therapy in OphthalmologyDocument11 pagesCurrent Status of The Use of Gene Therapy in OphthalmologyLiviu Athos TamasNo ratings yet
- Sudden Arrhythmic Death SyndromeDocument2 pagesSudden Arrhythmic Death SyndromeLiviu Athos TamasNo ratings yet
- Guidelines for Human Gene NomenclatureDocument14 pagesGuidelines for Human Gene NomenclatureLiviu Athos TamasNo ratings yet
- Irritable Bowel SyndromeDocument6 pagesIrritable Bowel SyndromeLiviu Athos TamasNo ratings yet
- Installation and Maintenance Guide: Applied Biosystems 7300/7500/7500 Fast Real-Time PCR SystemDocument178 pagesInstallation and Maintenance Guide: Applied Biosystems 7300/7500/7500 Fast Real-Time PCR SystemLiviu Athos TamasNo ratings yet
- Corneliu Omescu - Planeta Fara Memorie (1978) PDFDocument129 pagesCorneliu Omescu - Planeta Fara Memorie (1978) PDFClaudio FirulescuNo ratings yet
- Chaperoane 2005 Cystic FibrosisDocument11 pagesChaperoane 2005 Cystic FibrosisLiviu Athos TamasNo ratings yet
- El Cerebro Consume Lactato Durante El Ejercicio ExtenuanteDocument7 pagesEl Cerebro Consume Lactato Durante El Ejercicio ExtenuanteAlvaro PerezNo ratings yet
- CABDV CFTR DetectionDocument6 pagesCABDV CFTR DetectionLiviu Athos TamasNo ratings yet
- Actinomyces OdontolyticusDocument4 pagesActinomyces OdontolyticusLiviu Athos TamasNo ratings yet
- Mlpa 2007 CFTRDocument9 pagesMlpa 2007 CFTRLiviu Athos TamasNo ratings yet
- Diagnostic Molecular Fibroza CisticaDocument15 pagesDiagnostic Molecular Fibroza CisticaLiviu Athos TamasNo ratings yet
- 2006 Hypolipidemic DrugsDocument27 pages2006 Hypolipidemic DrugsLiviu Athos TamasNo ratings yet
- Biological Activities of Lupeol DesecurizatOKDocument21 pagesBiological Activities of Lupeol DesecurizatOKLiviu Athos TamasNo ratings yet
- MiRNA Profil Cancer SanDocument2 pagesMiRNA Profil Cancer SanLiviu Athos TamasNo ratings yet
- 2006 Number 1 Articol CFTRDocument6 pages2006 Number 1 Articol CFTRLiviu Athos TamasNo ratings yet
- Review EPCDocument14 pagesReview EPCLiviu Athos TamasNo ratings yet
- Profile Prince Decoware Furniture HandlesDocument54 pagesProfile Prince Decoware Furniture HandlesRushabh ShahNo ratings yet
- 11th Chemistry EM - Public Exam 2022 - Model Question Paper - English Medium PDF DownloadDocument3 pages11th Chemistry EM - Public Exam 2022 - Model Question Paper - English Medium PDF DownloadAshwini Shankar KumarNo ratings yet
- High Performance Butterfly Valve HP 111Document4 pagesHigh Performance Butterfly Valve HP 111JOHNNo ratings yet
- Lennox Kca090s4bn2yDocument36 pagesLennox Kca090s4bn2yGerardo ZamoranoNo ratings yet
- Index TO XV: ArsphenamineDocument7 pagesIndex TO XV: ArsphenamineAlex12No ratings yet
- Cementing Operation - Part IDocument26 pagesCementing Operation - Part IDoni KurniawanNo ratings yet
- Lesson 2 Dna Structure and Dna ExtractionDocument8 pagesLesson 2 Dna Structure and Dna ExtractionGreatel Elijah TorregosaNo ratings yet
- 1 Auxilliary Equipment - US PricingDocument132 pages1 Auxilliary Equipment - US PricingOscar EspitiaNo ratings yet
- 5 Steps Homemade BiogasDocument5 pages5 Steps Homemade BiogasJan Aguilar EstefaniNo ratings yet
- SDS Asam SulfatDocument8 pagesSDS Asam SulfatQuality AssuranceNo ratings yet
- Cniai 34686 0003Document45 pagesCniai 34686 0003Nirosha Dilrangi PereraNo ratings yet
- Acid RainDocument35 pagesAcid RainAshish Deotale100% (4)
- List I (16 5 2011)Document585 pagesList I (16 5 2011)Ayaz Mani50% (2)
- Properties of WaterDocument3 pagesProperties of WaterLupis HernándezNo ratings yet
- Gruvlok Stainless SteelDocument16 pagesGruvlok Stainless Steellink2u_007No ratings yet
- Vestamid L Polyamide 12 - EvonikDocument12 pagesVestamid L Polyamide 12 - EvonikHaryo Armono100% (1)
- II - ITP - Defect Work FabricationDocument5 pagesII - ITP - Defect Work Fabricationmohd as shahiddin jafriNo ratings yet
- SeminarDocument15 pagesSeminarAditi ChandraNo ratings yet
- Spectroscopy Primer AnswersDocument13 pagesSpectroscopy Primer AnswersjayshreeNo ratings yet
- Exporters: Index by CountryDocument113 pagesExporters: Index by CountrySyam WadiNo ratings yet
- SIP MeenakshDocument17 pagesSIP MeenakshRati GuptaNo ratings yet
- Thermoplastics and Thermosetting PlasticDocument24 pagesThermoplastics and Thermosetting PlasticKAPIL SINGHNo ratings yet
- Constant HeadDocument14 pagesConstant HeadfujiNo ratings yet
- Experiment 6 Lab ReportDocument21 pagesExperiment 6 Lab ReportmarkkkkkNo ratings yet
- A STUDY ON THE ECOFRIENDLY DYES EXTRACTED FROM THREE DIFFERENT SPECIES OF Curcuma LDocument3 pagesA STUDY ON THE ECOFRIENDLY DYES EXTRACTED FROM THREE DIFFERENT SPECIES OF Curcuma Lanon_285184956No ratings yet
- PVC FlamethrowerDocument21 pagesPVC FlamethrowerMike Nichlos100% (4)
- Big Batch Soap MakingDocument24 pagesBig Batch Soap MakingAnonymous Vu1R35s4WZ100% (2)
- Calculation Dilute Phase Pressure Drop Rhodes MethodDocument3 pagesCalculation Dilute Phase Pressure Drop Rhodes MethodBTENo ratings yet