Professional Documents
Culture Documents
AlSi Phase Diagram A
Uploaded by
Hellen-Xiujuan JiangOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
AlSi Phase Diagram A
Uploaded by
Hellen-Xiujuan JiangCopyright:
Available Formats
The Aluminum-Silicon Phase Diagram and Eutectic Modifications
Table 1: Preparation Method for Al-Si Specimens
Surface
CarbiMet 2 psa paper UltraPol silk cloth TriDent polyester cloth MicroCloth pad Deformed grain structure of 99.999% Al; Barkers Reagent, Polarized Light MicroCloth pad
Abrasive Size
220-320-grit SiC water cooled 9-m Diamond with MetaDi Fluid 3-m Diamond with MetaDi Fluid MasterMet Colloidal Silica MasterMet Colloidal Silica
Load Lb (N)
5 (22)
Platen Speed/Direction
200-240 rpm Contra 150 rpm Contra 150 rpm Contra 100-150 rpm Contra VibroMet 2 Vibratory Polisher
Time (min)
1 each
5 (22)
5-10
5 (22)
5-7
5 (22) -
3 30 Twins in polycrystalline 99.9999% Si, as-cast, aqueous 75% NaOH, Nomarski DIC
Reprinted with permission of ASM International All rights reserved. www.asminternational.org
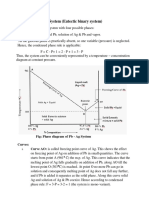
The Al-Si phase diagram is a straightforward, classic example of a eutectic system where each element has little, if any solubility in the other. Aluminum melts at 660.452 C while silicon melts at 1414 C. The diagram, from Murray and McAlister (Vol. 8, Metals Handbook, 8th ed., 1973, p. 263), shows the eutectic at 12.6 wt. % Si and 577 C. The maximum solubility of Si in Al is ~1.65% at 577 C, and the solubility decreases with decreasing temperature. There is virtually no solubility for Al in Si at any temperature to the melting point. The addition of silicon to aluminum improves fluidity; hence there are a number of commercially important alloys with ~7 wt. % Si. These alloys are used as sand castings or as permanent mold castings. There are also commercial alloys made at and above the eutectic Si content, mainly by injection molding, sometimes under pressure. These alloys solidify quickly and exhibit no long-range segregation. To obtain better properties, both hypoeutectic and quasi-eutectic alloys are modified by the addition of Sr or Na which affect the shape of acicular Si eutectic particles producing a globular shape. Phosphorous is added to quasi-eutectic and hypereutectic alloys so as to disperse and reduce the size of primary silicon cuboids. Ti and B are added to hypoeutectic alloys which decreases the size of primary -Al dendrites in hypoeutectic compositions. Faster cooling rates, as achieved by gravity or pressure die casting, promote greater than equilibrium amounts of proeutectic -Al and a finer eutectic particle spacing. Al 1% Si as-cast specimen with Si particles in an -Al matrix; Si Blue etch The table illustrates the procedure used to prepare the micrographs shown. While standard etchants, such as Kellers or 0.5% aqueous HF, can be used to reveal the Si particles, other reagents are more useful. Wecks reagent for Al (100 mL water, 4 g KMnO4 and 1 g NaOH) colors the -Al structure revealing segregation while the Si-Blue etch (90 mL water, 4 mL HF, 4 mL H2SO4, 2 g CrO3) will color the Si particles blue gray. Al 50% Si, as-cast, cracked primary Si and eutectic, Si Blue etch
Al 7.12% Si, as-cast, with primary -Al dendrites and an -Al/Si eutectic; Si Blue etch
Al 12% Si, as-cast, near eutectic, Si Blue etch
Na-Modified Al 12% Si, as-cast, near eutectic, Si Blue etch
Al 12.9% Si, gravity die cast, Si Blue etch
Al 25% Si, as-cast, hypereutectic, primary Si and an -Al/Si eutectic, Wecks Reagent
Al 11.7% Si, as-cast, with primary -Al dendrites and an -Al/Si eutectic; Si Blue etch
Al 12% Si, as-cast, near eutectic; Wecks Reagent, Polarized Light
Na-Modified Al 12% Si, as-cast, near eutectic, Wecks Reagent, Polarized Light
Al 12.9% Si 0.04% Sr, gravity die cast, Si Blue etch
Al 19.85% Si, as-cast, hypereutectic, primary Si plus eutectic, Wecks Reagent - eutectic cells, 200X
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Phase Equilibria 1: Problem Formulation (Chapter 6) : Live Your Life. Create Your DestinyDocument32 pagesPhase Equilibria 1: Problem Formulation (Chapter 6) : Live Your Life. Create Your DestinyNARE EDMUNDNo ratings yet
- CHE504 Heat and Mass Transfer LaboratoryDocument7 pagesCHE504 Heat and Mass Transfer LaboratoryAnis NazihahNo ratings yet
- Lesson Plan in ScinceDocument6 pagesLesson Plan in ScinceSheila Camba NarcaNo ratings yet
- 4 Ponchon Savarit MethodDocument47 pages4 Ponchon Savarit MethodDiogo HarduimNo ratings yet
- Kech 106Document47 pagesKech 106Ashutosh PantNo ratings yet
- Gen Chem2 Module Q1Week 3 4Document16 pagesGen Chem2 Module Q1Week 3 4Dan MacabingilNo ratings yet
- AFPX 407 Leaflet PDFDocument2 pagesAFPX 407 Leaflet PDFยุทธนา เทียมเมืองNo ratings yet
- LCSS Ethyl Acetate - CH3COOC2H5 - PubChemDocument66 pagesLCSS Ethyl Acetate - CH3COOC2H5 - PubChemwidyy universeNo ratings yet
- Equilibrium Phases in Cast AlloysDocument52 pagesEquilibrium Phases in Cast AlloysAkshayaa BalajiNo ratings yet
- OEUK Well Decommissioning CO2 Storage GuidelinesDocument52 pagesOEUK Well Decommissioning CO2 Storage GuidelinesMohammad AnnasNo ratings yet
- Gas Absorption Problem Set 2016Document2 pagesGas Absorption Problem Set 2016Jumar CadondonNo ratings yet
- Desulfurization of Synthetic SlagDocument10 pagesDesulfurization of Synthetic Slagjagadish mahataNo ratings yet
- Polymer Blend'sDocument18 pagesPolymer Blend'sMohsin Alam0% (1)
- Ure Substance: Saturation Temperature (T)Document13 pagesUre Substance: Saturation Temperature (T)Muhammad MasroorNo ratings yet
- The Science of Soap Films and Soap Bubbles (Cyril Isenberg)Document219 pagesThe Science of Soap Films and Soap Bubbles (Cyril Isenberg)adamrou100% (11)
- Andrew's ExperimentDocument7 pagesAndrew's ExperimentPragyan ChutiaNo ratings yet
- HANDOUT in PHYSICS - Heat, Specific Heat Phase Change, and CalorimetryDocument5 pagesHANDOUT in PHYSICS - Heat, Specific Heat Phase Change, and CalorimetryJonathan Briones Mses MsphyNo ratings yet
- BakeliteDocument43 pagesBakeliteMuhammad Irfan MalikNo ratings yet
- Mesomorphic Phases and Phase Transitions in Membranes, Biomechanical AspectsDocument18 pagesMesomorphic Phases and Phase Transitions in Membranes, Biomechanical AspectsTatiana AndradeNo ratings yet
- SPE 62933 The Relative Significance of Positive Coupling and Inertial Effects On Gas Condensate Relative Permeabilities at High VelocityDocument10 pagesSPE 62933 The Relative Significance of Positive Coupling and Inertial Effects On Gas Condensate Relative Permeabilities at High VelocityAndrés Eduardo Guzmán VelásquezNo ratings yet
- Thermodynamics Temperature08Document19 pagesThermodynamics Temperature08Abdelkader Faklani DouNo ratings yet
- Summary SectionDocument71 pagesSummary SectionAhmed Khalil JaberNo ratings yet
- Lecture 08 Phase Diagram Type III GroupDocument20 pagesLecture 08 Phase Diagram Type III GroupAhmad NawazNo ratings yet
- PB Ag SystemDocument2 pagesPB Ag SystemAtul Gautam100% (2)
- Liquid-Liquid Equilibrium For Ternary System Containing Biodiesel, Methanol and WaterDocument8 pagesLiquid-Liquid Equilibrium For Ternary System Containing Biodiesel, Methanol and WaterAdani Ghina Puspita SariNo ratings yet
- Experiment #3: Sublimation and Melting Point DeterminationDocument3 pagesExperiment #3: Sublimation and Melting Point DeterminationMarthy DayagNo ratings yet
- The Isothermal Van't Hoff Equation For Phase Equilibria-A Forgotten Relation?Document6 pagesThe Isothermal Van't Hoff Equation For Phase Equilibria-A Forgotten Relation?JESUS DAVID BOLA‹O JIMENEZNo ratings yet
- Sun 2019Document8 pagesSun 2019Khairool Azizul MohammadNo ratings yet
- Phase Changes in MatterDocument29 pagesPhase Changes in MatterLindsay CrystalNo ratings yet
- Refrigerant Inventory TechNoteDocument36 pagesRefrigerant Inventory TechNotedhavaleshNo ratings yet