Professional Documents
Culture Documents
Krunal Vora Content
Uploaded by
Devashish JoshiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Krunal Vora Content
Uploaded by
Devashish JoshiCopyright:
Available Formats
201 1
STAINLESS STEEL PLATING
(ALLOY PLATING)
KRUNAL VORA--------947
SAMSUNG DEPARTMENT OF METALLURGICAL & MATERIAL SCIENCE ENGINEERING. FACULTY OF TECHNOLOGY & ENGINEERING. THE M.S.UNIVERSITY OF VADODARA. 9/26/2011
PLATING
Plating is a surface covering in which a metal is deposited on a conductive surface. Plating has been done for hundreds of years, but it is also critical for modern technology. Plating is used to decorate objects, for corrosion inhibition, to improve solder ability, to harden, to improve wear ability, to reduce friction, to improve paint adhesion, to alter conductivity and for many other purposes. Jewelry typical uses plating to give a silver or gold finish. Thin film deposition has plated objects as small as an atom; therefore plating finds in NANO TECHNOLOGY. There are several plating methods, and many variations. In one method, a solid surface covered with metal sheet, and then heat and pressure are applied to fuse them. Other plating techniques vapor deposition under vacuum and sputter deposition. Recently, plating often refers to using liquids. Metalizing refers to coating metal on non-metallic objects.
ELECTROPLATING
In electroplating, an ionic metal is supplied with electrons to form a non-ionic coating on a substrate. A common system involves a chemical solution with the ionic form of the metal, an anode which may consist of the metal being plated or an insoluble anode, and finally, a cathode where electrons are supplied to produce a film of no- ionic metal
Electroplating is used in many industries for functional & decorative purpose .Some well- known examples for chrome-plating of steel parts on automobiles. Steel bumpers become more corrosion-resistant when they have been electroplated with first Nickel and then Chromium.
Hard chromium is used in services where frictional wear must be a minimum, such as hydraulic pistons & camshaft bearing diameters.
Plain steel or aluminum parts in light fixtures glisten when they are electroplated with nickel and then decorated with chromium or brass.
Nickel, in the form of a nickel sulfate, is used to restore dimensions on worn parts, and as under the plate for hard chrome. The nickel sulfate bath is unsuitable for decorative work.
Steel bolts last much longer because they are sold with a coating of zink or cadmium that has been applied by electroplating. These electroplating and conversion coating provide a double protection system for steel components.
Virtually all types of steel can be protected including casting. Newly developed electrolytes and process methods are able to provide greatly increased corrosion prevention and brilliant finishes. Specially developed process produce improved metal distribution over complex shapes.
ALLOY PLATING
In some cases , it is desirable to co-deposit two or more metals resulting as an electroplated deposit. Depending on the alloy system, an electroplated alloy may be solid solution strengthened or precipitation hardened by heat treatment to improve the platings physical and chemical properties. Nickel-Cobalt is a common electroplated alloy. Nickel-Cobalt is a common electroplated alloy.
PROCESS OF PLATING
Three main stages of alloy deposition are,
1 .IONIC MIGRATION:
The hydrated ions in electrolyte migrate towards the cathode under the influence if applied potential as well as through diffusion and/or convention.
2. ELECTRON TRANSFER:
At the cathode surface are, the hydrated metal ions enter the diffusion double layer where the water molecules of the hydrated ions are aligned by the field present in this layer. Subsequently the metal ions enter the fixed double layer where because the higher field present, the hydrated shell is lost. Then on the cathode surface , the individual ion may be neutralized and is absorbed.
3. INCORPORATION:
The absorbed atom wonder to a growth point on the cathode and is incorporated in the growing lattice.
PURPOSE OF ALLOY PLATING:
1. Denser and finer grained
Electro deposited alloys are denser and finer grained than their constituent metal deposited under similar condition.
2. Decorative purpose
For example, gold alloys of various colures are used in jewelry. Cu - Son alloys has silvery white appearance and therefore can be used for decorative purposes.
3. Resistance to wear and abrasion
Electro-deposited alloys offer advantages in respect to resistance to wear and abrasion. E.g. Tin bronze which is harden than silver.
4. Protective purpose
Alloy deposition has great application for protective purposes because of its larger corrosion resistance. Tin-nickel & Cu- Son alloys have got extreme resistance to tarnishing.
5. Permits depositions of metal that cannot be plated as single metal
Co which cannot be plated from aqueous solutions. But it can be deposited as alloy
Stainless steel. What is it ?
The term "stainless" was used initially in the development of the steel for the cutlery business in the Production of knives, forks, spoons etc. There are now many stainless steel types and grades used in applications requiring resistance to staining, oxidation and general corrosion resistance. The main element of stainless steel is iron (Fe) hence it is an iron alloy containing a minimum of 12% chromium. Additional alloying elements are added to provide strength, cold working ability and toughness for example:o o o o o Copper Molybdenum Titanium Nickel Other non-metals are added primarily Nitrogen and Carbon.
The main objective in the selection of a type and grade of stainless steel is to meet the Performance requirements for strength and durability in its particular intended application. The two common grades of stainless steel used for construction fixings in the stone and masonry sectors are from the Austenitic group commonly referred to as follows:Grade 304 or Grade 316 Grade 304 is generally specified for normal environmental applications. Grade 316 is specified where a higher level of corrosion resistance is required e.g. marine environment.
Why choose stainless steel?
The cost of stainless steel is very competitive compared with other nickel or titanium based alloys and offers a range of corrosion resistant properties suitable for large number of industrial applications. Their strength is superior to plastics materials stainless steel can be hot or cold worked and fabricate using standard and traditional engineering techniques. Unlike certain plastics stainless steel is fully recyclable.
What makes stainless steel "stainless?
The reason for the high level of corrosion resistance of stainless steel is due to its self repairing properties. This is achieved by the formation of naturally occurring chromium-rich "passive" chromium oxide film on the surface of the steel. The film is extremely adherent and chemically stable providing sufficient oxygen is available to the surface for the chromium rich oxide film to form. It is important to remember that stainless steel can corrode under certain conditions and it is essential therefore to select the correct grade for a given application.
Stainless steel and its corrosion and oxidation resistance.
As a general rule, the higher the chromium content of stainless steel the higher the corrosion resistance. When nickel is added to create the austenitic steel the oxide film is strengthened and increases the durability in more aggressive environments. If molybdenum is added to either austenitic or ferrite stainless steel the pitting corrosion resistance is greatly improved..
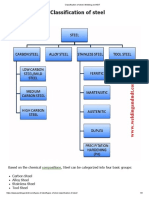
The family group of stainless steels.
Stainless steels are divided into four main group types namely Austenitic, Ferrite, Martensitic and Duplex. These names describe the crystalline structure of the steel
AUSTENITIC STAINLESS STEEL
Austenitic stainless steels have typically 18% chromium and contain nickel. This changes the metallic structure from ferritic to austenitic and improves the corrosion resistance. They cannot be hardened by heat treatment. They are non-magnetic however a small amount of local magnetism is produced after cold forming such as bending and rolling.
FERRITIC STAINLESS STEEL
Ferritic stainless steels contain chromium as their main alloying element of between 13% - 17% and have a low carbon content. They are magnetic and cannot be hardened by heat treatment.
MARTENSITIC STAINLESS STEEL
Martensitic stainless steels have typically 12% chromium with higher carbon content than the ferritic types. They are magnetic and can be hardened by normal quenching and tempering techniques like medium and high plain carbon steels. Martensitic steels are commonly used in the production of cutlery and are also used in the aerospace industry.
DUPLEX STEEL
Duplex stainless steels are used where both corrosion resistance and strength are required. They cannot be hardened by heat treatment. Their metallic structure is a combination of austenite and ferrite. "Super" Austenitic and "Super" Duplex stainless steels have a greater resistance to pitting or crevice corrosion-than the ordinary austenitic or duplex steels. This is due to additional chromium, nitrogen and molybdenum. Precipitation Hardening stainless steels can be hardened and strengthened like the martensitic group by heat treatment. The metallic structure has a different mechanism to the process of the martensitic types consequently either austenitic or martensitic precipitation hardening structures can be formed.
International material Standards: EN, ASTM and JIS
EN 10088-2 (Previously UK BS 1449 part 2) Stainless steel - Sheet, plate and strip for
general purposes. EN 10151- Stainless steel strip for springs. ASTM A240- Heat-resisting Cr and Cr-Ni stainless steel plate, sheet and stripfor pressure vessels. ASTM A167- Stainless and heat-resisting Chromium-Nickel steel plate, sheet and strips ASTM A176- Stainless and heat resisting Chromium steel plate, sheet and strip. ASTM A666 -Austenitic stainless steel sheet, strip, plate, bar for structural And architectural applications.
JIS G4308 -Stainless steel wire rods. JIS G4303- Stainless steel bars
STAINLESS STEEL PLATING
In stainless steel main alloying element is Chromium(Cr) and Nickel(Ni) and it is a alloy of Iron(Fe). So here we study about the chromium plating
CHROMIUM PLATING
It is widely used for the coating of steel. Electrolytically deposited chromium coating can have a morror finish and are of a silver - colour with a bluish tinge The potential of chromium is more electronegative than that of iron but owing to the strong tendency of chromium to become passive in air, coating of this metal protect steel only mechanically.
METHODS FOR PRODUCTION OF CHROMIUM COATING:
Chromium cementation OR chromizing. Electrodeposition.
CHROMIZING>>>>>
It is classified into 3 types Powder process vapour process fused salt process It is known as enrichment of the surface region of steels with chromium by thermochemical treatment. During this treatment chromium atoms diffuse at temperature between 900 and 1000 C into the surface of the workpiece.
ELECTRODEPOSITION
Chromium plating has found widespread use in two different field of application 1.Decorative Cr plating 2.hard Cr plating Decorative Cr plating: thin bright coating 0.002 to 0.020 mil in thickness is used over nickel or copper undercoat. Bright finish of moderate durability is obtained Hard Cr plating: it is directly applied to steel, without any intermediate deposit of other mrtals. The thickness of the chromium coating can very from 0/075 to 0.25 milimeter, but range can range from 0.005 to 0.01 mil.it is deposited for high hardness, wear resistance & low coefficient of friction. After such treatment the service life of steel articles is considerably increased.
PROPERTIES OF CHROMIUM DEPOSIT:
a) b) c) d) e) f) 1.It has a characteristic bluish white tint 2.Shows unusual resistance to oxidation and tarnish 3.Hardness of chromium deposit is of the order of 900 BHN. 4.low coefficient to friction 5.Not affected by alkalies , sulphur & its compounds etc. 6.It is non magnetic.
ELECTROLYTIC BATH
Chromic-acid-based bath (Hexavalent Type)
In plating from this bath, sulfate and fluoride ions act as catalysts. Temperature , current density and bath composition affect the film characteristics and current efficiency. Thickness of deposits vary from 0.25 to 0.5 micron(0.001 inch), or more for hard chromium plating.
Bath Composition
Chromic acid and sulfate are the necessary ingredients. Chromic-to-sulfate ratio range from 50:1 to 250:1. Preferably at 100:1. The composition depends on whether the bath is cocatalyzwd, e.g.with fluorides, fluosilicates or fluoborates, and on the application.
Basically 2 types of baths commonly used 1.Concentrated solution bath containing CHROMATE(Cr2O3) 400g/1 & H2SO4 4g/1 Advantage -higher conductivity and lesser voltage required rate of deposition becomes faster Disadvantage Low throwing power Narrow range of current density for bright deposit
2.Dilute solution bath containing Cr2O3 250g/1 & H2SO4 4g/1 Advantage Less costlier Wide range of current density for bright deposit Disadvantage Low conductivity Requires high voltage
FACTOR AFFECTED ON THE PLATING
1. TEMPERATURE
It is closely related to current density in its effect on brightness and coverage of deposit. Generally, the brighter the current density, the higher temperature requirement. For decorative bath the range is 35C to 46C & for hard chromium, the range is 49C to 65.5C Agitation is required to equalize the bath temperature, to produce uniform brightness, and in the case of hard chromium, to improve deposit hardness. Preheating of parts to optimum bath temperature may be needed before they introduced into the tank amd in rare case cooling of parts may be needed
2. CURRENT DENSITY
At given solution composition and temperature, current density affects cathode efficiency, brightness and hardness. Generally optimum current density is recommended. At too high current densities, burning or roughness of deposit occurs. At low current densities, lack of chromium coverage can be expected. The increase in current density or decrease in it depending on whether the temperature is below or above 55C. At 55C hardness is independent of current density.
3. CURRENT EFFICIENCY
It depends on temperature and current density. It decrease with increasing current and increase exponentially with increase in current density.
4. SOLUTION DEPOSITION
Increasing the chromic acid concentration reduces the rate of deposition. Increasing chromic acid concentration or sulfate keeping the chromic/sulphuric acid ratio constant decrease the hardness of deposit.
5. ANODES
Iron anode are used in stainless steel plating.
COMMON PLATING PROBLEM
Faulty bath chemistry Improper temperature and/or current density
Poorly finished basis-metal surface
TYPICAL APPLICATIONS
Printing plates Wrist pins Steering Knuckles Tube drawing dies Forming dies water pipes surgical applications Architectural
WORLD WIDE APPLICATIONS
Sant Fruitos pedestrian bridge in SPAIN
Padre arrupe bridge in SPAIN
REFERENCES
Google search
www.scrabd.com www.4shared.com www.wikipedia.org sharan and narain
THANK
YOU
You might also like
- Stainless Steel MaterialDocument9 pagesStainless Steel MaterialdeliNo ratings yet
- Stamping 101: Material Guidelines: Properties and Characteristics That Affect FormabilityDocument5 pagesStamping 101: Material Guidelines: Properties and Characteristics That Affect FormabilityDavid RodriguezNo ratings yet
- The Stainless Steel Family - An Overview - Campbell Tip of The MonthDocument5 pagesThe Stainless Steel Family - An Overview - Campbell Tip of The Monthpeach5No ratings yet
- Complete Guide to Stainless Steel Properties and ApplicationsDocument16 pagesComplete Guide to Stainless Steel Properties and ApplicationsRahul SinghNo ratings yet
- Final MT-4 & 5Document31 pagesFinal MT-4 & 5RajasekharKosuruNo ratings yet
- Types of corrosion and protective coatings for process equipmentDocument3 pagesTypes of corrosion and protective coatings for process equipmentakshaylattimardiNo ratings yet
- Lesson Title: Types of MetalsDocument8 pagesLesson Title: Types of MetalsEric LamNo ratings yet
- Stainles SteelDocument66 pagesStainles SteelHarshita DabasNo ratings yet
- U030 enDocument28 pagesU030 enkiran_wakchaureNo ratings yet
- GCE3135 AssignmentDocument10 pagesGCE3135 AssignmentBilal MishoryNo ratings yet
- HeliCoil Technical Information Corrosion Screw ThreadsDocument6 pagesHeliCoil Technical Information Corrosion Screw ThreadsAce Industrial SuppliesNo ratings yet
- An Introduction To Stainless SteelsDocument5 pagesAn Introduction To Stainless SteelsMELVIN MAGBANUANo ratings yet
- By - Shaik ShahidDocument22 pagesBy - Shaik ShahidAnonymous q6SfMddJDoNo ratings yet
- Bme - Part 1Document49 pagesBme - Part 1Sumanth ChallaNo ratings yet
- Need of Surface TreatmentDocument6 pagesNeed of Surface TreatmentRahul MoottolikandyNo ratings yet
- Carbon steelDocument9 pagesCarbon steelArfanAliNo ratings yet
- Stainless Steel ClassificationDocument5 pagesStainless Steel Classificationyatin888No ratings yet
- MZ FS Unit - 1Document27 pagesMZ FS Unit - 1Jai KumarNo ratings yet
- Overview of StainlessDocument1 pageOverview of Stainlessnrd9771No ratings yet
- Workshop ReportDocument8 pagesWorkshop ReportAloshNo ratings yet
- Material Science: Prof. Satish V. KailasDocument12 pagesMaterial Science: Prof. Satish V. KailasAlvin SmithNo ratings yet
- Define Stainless OKDocument12 pagesDefine Stainless OKabasoudaNo ratings yet
- NON Ferrous AlloysDocument37 pagesNON Ferrous AlloysRajan ChaudharyNo ratings yet
- MetalsDocument58 pagesMetalsAkanksha VermaNo ratings yet
- Engineering Materials I Notes 2022 METALSDocument15 pagesEngineering Materials I Notes 2022 METALSAliciaNo ratings yet
- Technical Update How To Weld Maintain Stainless SteelDocument16 pagesTechnical Update How To Weld Maintain Stainless Steeloquintero99No ratings yet
- Proteksi InggrisDocument9 pagesProteksi Inggrisbo_lankNo ratings yet
- Alloy Steel and Cast IronDocument16 pagesAlloy Steel and Cast IronDennis AlvarezNo ratings yet
- Galvanic Series: Why Metals Corrode?Document7 pagesGalvanic Series: Why Metals Corrode?Rey Francis FamulaganNo ratings yet
- Stainless SteelDocument4 pagesStainless SteelMARUCOT ALEXIS P.No ratings yet
- Application of Stainless SteelDocument13 pagesApplication of Stainless Steelsweety1188No ratings yet
- 202 Out1Document5 pages202 Out1professorNo ratings yet
- Comsats University Islamabad, Lahore CampusDocument4 pagesComsats University Islamabad, Lahore CampusMaryam FatimaNo ratings yet
- Welding of Stainless SteelsDocument48 pagesWelding of Stainless SteelsRamzi BEN AHMEDNo ratings yet
- Stainless Steels Written ReportDocument13 pagesStainless Steels Written ReportSteve manicsicNo ratings yet
- Stainless SteelDocument24 pagesStainless SteelsmrutiNo ratings yet
- Imp NotesDocument7 pagesImp Notes22102048No ratings yet
- Austenitic Stainless SteelsDocument10 pagesAustenitic Stainless SteelsbramNo ratings yet
- Aircraft Material and ProcessesDocument392 pagesAircraft Material and ProcessesDavid John Balanag100% (4)
- Ferrous Metals and AlloysDocument44 pagesFerrous Metals and AlloysLeonardDacaymatNo ratings yet
- Module 5: Aircraft General Standards: Engineering MaterialsDocument34 pagesModule 5: Aircraft General Standards: Engineering MaterialsmarshallNo ratings yet
- METALLOGRAPHIC PROPERTIES: MILD STEELS TO BRASSDocument4 pagesMETALLOGRAPHIC PROPERTIES: MILD STEELS TO BRASSmuralidharanNo ratings yet
- Chapter 5 - Metal AlloysDocument75 pagesChapter 5 - Metal AlloysAnonymous LSRTDBL100% (1)
- Ferrous-Non Ferrous and Corrosion - 11!08!2011Document6 pagesFerrous-Non Ferrous and Corrosion - 11!08!2011Rohan RamguttyNo ratings yet
- Metals 2Document13 pagesMetals 2arooj anjumNo ratings yet
- Chap-10 Materials and Fabrication SelectionDocument51 pagesChap-10 Materials and Fabrication SelectionSuprio KamalNo ratings yet
- Material EngineeringDocument46 pagesMaterial EngineeringBoaquin KhenNo ratings yet
- Stainless Steel 6Document10 pagesStainless Steel 6YKAGARWALNo ratings yet
- KNS1042 Metals Part1 W8Document29 pagesKNS1042 Metals Part1 W8justine2109No ratings yet
- Metals Classification and Properties GuideDocument40 pagesMetals Classification and Properties Guideaman sudiNo ratings yet
- Stainless SteelDocument7 pagesStainless SteelRathnakrajaNo ratings yet
- Why Stainless Steel for Rail CoachesDocument83 pagesWhy Stainless Steel for Rail CoachesaravindanNo ratings yet
- Steel and Steel Making: Fact: Carbon Steels Make Up About 90% of All Steel ProductionDocument6 pagesSteel and Steel Making: Fact: Carbon Steels Make Up About 90% of All Steel ProductionSAMANTHA SARAH PURBANo ratings yet
- Materials QuestionsDocument30 pagesMaterials QuestionsMatheus SouzaNo ratings yet
- Stainless Steel Electrical ApplicationsDocument9 pagesStainless Steel Electrical ApplicationsUmang SoniNo ratings yet
- INSDAG - Institute for Steel Development and GrowthDocument3 pagesINSDAG - Institute for Steel Development and GrowthJeeva Z FedricoNo ratings yet
- Aalco MaterialsDocument20 pagesAalco MaterialsMohamed FaragNo ratings yet
- Chapters On Nonferrous MetalsDocument17 pagesChapters On Nonferrous MetalsMohmmad ShaikhNo ratings yet
- Engineering Materials: Metals and Their Alloys Ceramics Polymers CompositesDocument53 pagesEngineering Materials: Metals and Their Alloys Ceramics Polymers CompositesSyed Muhammad AliNo ratings yet
- Seminar ON Significance of H - H O-O Lines in Pourbaix DiagarmDocument10 pagesSeminar ON Significance of H - H O-O Lines in Pourbaix DiagarmDevashish JoshiNo ratings yet
- Vipul Mevasiya ContentDocument15 pagesVipul Mevasiya ContentDevashish JoshiNo ratings yet
- Detecting Susceptibility To Intergranular Corrosion: Seminar ONDocument12 pagesDetecting Susceptibility To Intergranular Corrosion: Seminar ONDevashish JoshiNo ratings yet
- Ronak Mehta ContentDocument12 pagesRonak Mehta ContentDevashish JoshiNo ratings yet
- Gaurav Chudasama ContentDocument11 pagesGaurav Chudasama ContentDevashish JoshiNo ratings yet
- Nidhi Shah ContentDocument6 pagesNidhi Shah ContentDevashish JoshiNo ratings yet
- Mitesh ContentDocument18 pagesMitesh ContentDevashish JoshiNo ratings yet
- A Seminar On Cathodic Protection TechniquesDocument9 pagesA Seminar On Cathodic Protection TechniquesDevashish JoshiNo ratings yet
- Sumit ContentDocument6 pagesSumit ContentDevashish JoshiNo ratings yet
- Types of Methods of Powder Production:: Physico Chemical Processes Are As UnderDocument11 pagesTypes of Methods of Powder Production:: Physico Chemical Processes Are As UnderDevashish JoshiNo ratings yet
- Dinkar Kokje ContentDocument8 pagesDinkar Kokje ContentDevashish JoshiNo ratings yet
- A Seminar On Electrolytic Production of Metallic Powder: Prepared By: Darshit Fadadu ROLL NO: 938Document13 pagesA Seminar On Electrolytic Production of Metallic Powder: Prepared By: Darshit Fadadu ROLL NO: 938Devashish JoshiNo ratings yet
- Parth ContentDocument9 pagesParth ContentDevashish JoshiNo ratings yet
- Electric Discharg Machining: Parikh Krutik R. ROLL NO.931Document6 pagesElectric Discharg Machining: Parikh Krutik R. ROLL NO.931Devashish JoshiNo ratings yet
- Metallic Coatings For Corrosion PreventionDocument9 pagesMetallic Coatings For Corrosion PreventionDevashish JoshiNo ratings yet
- Prevention From Inter-Granual CorrosionDocument7 pagesPrevention From Inter-Granual CorrosionDevashish JoshiNo ratings yet
- Ankur ContentDocument6 pagesAnkur ContentDevashish JoshiNo ratings yet
- Corrosion in Various Environments and Their RoleDocument9 pagesCorrosion in Various Environments and Their RoleDevashish JoshiNo ratings yet
- Arjun ContentDocument13 pagesArjun ContentDevashish JoshiNo ratings yet
- Seminar Topic On Galvanic Corrosion ParametersDocument6 pagesSeminar Topic On Galvanic Corrosion ParametersDevashish JoshiNo ratings yet
- Anil Vaghamshi ContentDocument6 pagesAnil Vaghamshi ContentDevashish JoshiNo ratings yet
- Electrometallurgy and Corrosion Seminar On Fuel Cells: Name: Anirudh Gupta ROLL NO.: 902 YEAR: 2011Document10 pagesElectrometallurgy and Corrosion Seminar On Fuel Cells: Name: Anirudh Gupta ROLL NO.: 902 YEAR: 2011Devashish JoshiNo ratings yet
- VaishaliDocument15 pagesVaishaliDevashish JoshiNo ratings yet
- VibhanshuDocument21 pagesVibhanshuDevashish JoshiNo ratings yet
- Fe-H2O System in Pourbaix Diagram: Seminar Topic OnDocument21 pagesFe-H2O System in Pourbaix Diagram: Seminar Topic OnDevashish JoshiNo ratings yet
- SUMITDocument27 pagesSUMITDevashish JoshiNo ratings yet
- Tushal KyadaDocument11 pagesTushal KyadaDevashish JoshiNo ratings yet
- TrushitDocument22 pagesTrushitDevashish JoshiNo ratings yet
- Ronak MehtaDocument17 pagesRonak MehtaDevashish JoshiNo ratings yet
- The Economic Benefits of Chlorine Chemistry in Titanium and Titanium Dioxide in The US and Canada PDFDocument15 pagesThe Economic Benefits of Chlorine Chemistry in Titanium and Titanium Dioxide in The US and Canada PDFAmer AlkalaifhNo ratings yet
- Chemical Reactions and EquationsDocument13 pagesChemical Reactions and Equationsprodigypls100% (1)
- Weldability of SteelsDocument5 pagesWeldability of SteelsKhalid El Masry100% (1)
- Fiberglass Products: Product Line Overview & CapabilitiesDocument16 pagesFiberglass Products: Product Line Overview & CapabilitiesJairo FigueroaNo ratings yet
- Material Science Question BankDocument4 pagesMaterial Science Question BankpramodNo ratings yet
- Steel Alloys Guide for Heavy-Duty CastingsDocument6 pagesSteel Alloys Guide for Heavy-Duty CastingsSanthosh LingappaNo ratings yet
- 1 s2.0 0304386X9190055Q MainDocument32 pages1 s2.0 0304386X9190055Q MainJordan Ulloa Bello100% (1)
- AS-A Level Geology: The Goldschmidt SystemDocument11 pagesAS-A Level Geology: The Goldschmidt SystemBikash MohantyNo ratings yet
- Steel vs Aluminum - Weight, Strength & Cost ComparisonDocument3 pagesSteel vs Aluminum - Weight, Strength & Cost ComparisonchungNo ratings yet
- Why You Should Use Mercedes-Benz’s New Blue Anti-FreezeDocument6 pagesWhy You Should Use Mercedes-Benz’s New Blue Anti-FreezepaufabraNo ratings yet
- D and F Block ElementsDocument18 pagesD and F Block ElementsLakshmi SinghNo ratings yet
- Blacksmith IngDocument14 pagesBlacksmith IngKhatty CutamoraNo ratings yet
- Design of Joints: Equation Chapter 1 Section 1Document131 pagesDesign of Joints: Equation Chapter 1 Section 1kartikijag100% (1)
- Electricity and Chemistry - QPDocument29 pagesElectricity and Chemistry - QPEman AbdellatifNo ratings yet
- SAC ALLOY 305 MaterialDocument2 pagesSAC ALLOY 305 Materialmuki10No ratings yet
- Classification of Steel - Welding and NDTDocument3 pagesClassification of Steel - Welding and NDTAshif Iqubal100% (1)
- 8 - Chapter 11-1Document33 pages8 - Chapter 11-1JCNo ratings yet
- Mole ConceptDocument52 pagesMole ConceptRekha Purohit60% (5)
- D and F Block Elements 2Document9 pagesD and F Block Elements 2Dr. P.S.SenguptaNo ratings yet
- Qualitative AnalysisDocument9 pagesQualitative AnalysisMaria Pauline Sarmiento GonzalesNo ratings yet
- Hypo Handbook Oxy ChemDocument20 pagesHypo Handbook Oxy ChemMaria Jose100% (2)
- Classification of Mineral Deposits TypesDocument5 pagesClassification of Mineral Deposits TypesJustin HernandezNo ratings yet
- 1 s2.0 S0169136814001553 MainDocument24 pages1 s2.0 S0169136814001553 MainDiana RochaNo ratings yet
- Formula Costo SandblastingDocument20 pagesFormula Costo SandblastingSerch VillaNo ratings yet
- Manganese Phosphate Coating AnalysisDocument23 pagesManganese Phosphate Coating AnalysisdamianNo ratings yet
- CH205 Lab - Corrosion (S11172685)Document6 pagesCH205 Lab - Corrosion (S11172685)Nitesh ChandNo ratings yet
- Iron Ore Jigging PlantDocument2 pagesIron Ore Jigging PlantryithanNo ratings yet
- Periodic Table of ElementalsDocument1 pagePeriodic Table of ElementalsXhian TadzNo ratings yet
- 3 Career EpisodeDocument9 pages3 Career Episodeashfaqur_rahman100% (5)
- Aragonite gemstone meaning and propertiesDocument3 pagesAragonite gemstone meaning and propertiesAstro GouravNo ratings yet