Professional Documents
Culture Documents
Test 01 Spring 07
Uploaded by
amiraliiiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Test 01 Spring 07
Uploaded by
amiraliiiCopyright:
Available Formats
First Exam Chemistry 3332 February 16, 2007
Name (PRINT)
.
Signature ID#
Last, First
Please circle class time.
Dr. Beans 10:00 AM Dr. Beans 1:00 PM
Page # 1. 12 pts. 2. 18 pts. 3. 18 pts. 4. 12 pts. 5. 14 pts. 6. 14 pts. 7. 12 pts.
. Score
TOTAL
Note: Present your student ID when you return the exam booklet
A. Nomenclature: (12 points) Give an acceptable name for each of the following compounds. Be sure to indicate the stereochemistry where appropriate.
Br
OCH3
O OH
2
CH2CH3
3
O
B. Facts: Total Points = 18 1. Place the following alkenes in order of increasing stability. (1=least stable, 3=most stable) (3 pts.)
2. Place the following carbocations in order of increasing stability. (1=least stable, 3=most stable) (3 pts.)
CH3 CH3 O CH2CHCH2
CH3 CH2 O CH2CHCH3
CH3 O
CH3 CH2CCH3
3. Place the following molecules in order of increasing reactivity in a Diels-Alder reaction. (1=least reactive, 3=most) (3 pts.)
OCH3 H H OCH3
H OCH3 CH3O H
H OCH3
H OCH3
4. Put the letter of the reaction with the faster rate in the box. (4 pts.)
a
Cl
NaCN CN NaCN Cl CN
5. Answer the following questions for the molecule below and place the answers in the appropriate boxes. (i)How many distinct types of protons are present in the molecule? (ii) How many distinct carbons are present? (iii) & (iv) What are the theoretically predicted multiplicities (splitting patterns) of the signal for protons b and c? (v) What is the multiplicity of carbon a in the proton-coupled 13C NMR? (5 pts.) (i) # of proton types
CH3 CH3CHCH2CH2 O H H
(ii) # of carbon types
b
(iii) multiplicity of Hb (iv) multiplicity of Hc (v) multiplicity of Ca
H3C
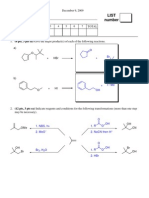
C. Reactions: Total = 30 points, 6 points each Please provide the major product in the answer box unless indicated otherwise. Indicate stereochemistry with wedges and dashes if applicable. Partial credit is awarded only when intermediate products in a multistep reaction are shown below the reaction.
1.
Br CH2
Br CHCH2 CH3
1. KOH / 200C 2. Na / NH 3 3. Br 2 / H2 O 4. NaOH
2.
Br
1. CH3 O- Na+
CH3 (H3C)3C
2. O3 3. (CH3 )2 S 4. NaBH4 / EtOH
3.
CH3O
Heat
4.
NBS / light / 0C +
Note: NBS=N-bromosuccinimide
Major Product
Minor Product
5.
CH3CH2CH C CH3 CH3
1. MCPBA 2.
MgBr
3. H 2 SO 4 / Heat
D. Mechanisms: (14 points) Provide a clear mechanism to explain the formation of the product. Use curved arrows to indicate electron flow. Remember to show only one step at a time. Show all intermediates and all formal charges. Do not show transition states!
Br Br2 / H2O
OH
E. Synthesis: (14 points) Synthesize the molecule below using any of the following reagents: alcohols, alkanes, alkenes and/or alkynes of three carbons or less, any inorganic reagents, any oxidizing or reducing agents, and any peroxyacids.
CH3CH2O
F(a). Spectroscopy (12 points) A compound with the formula C10H10O exhibits the IR, 1H NMR and proton decoupled 13C NMR spectra shown below. Please identify this compound d and draw the structure in the box provided below.
You might also like
- Please Circle Class Time.: Dr. Bean's 10:00 AM Dr. Bean's 1:00 PMDocument11 pagesPlease Circle Class Time.: Dr. Bean's 10:00 AM Dr. Bean's 1:00 PMAlex JohnsonNo ratings yet
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNo ratings yet
- 2423 e 2Document24 pages2423 e 2Agustin KurniatiNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- CHEM2700 Exam Answer - 2007W 2nd MidtermDocument11 pagesCHEM2700 Exam Answer - 2007W 2nd MidtermBruce CourtinNo ratings yet
- CHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsDocument15 pagesCHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsSara Yuen100% (1)
- 232 F 2010 Practice Midtem 3Document14 pages232 F 2010 Practice Midtem 3Chemist MeNo ratings yet
- Version Practice : Chemistry 1041 Practice Exam 2.1 Dr. WaddellDocument8 pagesVersion Practice : Chemistry 1041 Practice Exam 2.1 Dr. WaddelljamalNo ratings yet
- 231 F 2010 Practice MT4 - Pp1to12Document12 pages231 F 2010 Practice MT4 - Pp1to12Chemist MeNo ratings yet
- Practice Exam 2.3-1Document6 pagesPractice Exam 2.3-1jamalNo ratings yet
- Test1 342 PracticeV1Document5 pagesTest1 342 PracticeV1Camha NguyenNo ratings yet
- Honor Code: by The Definition of Academic Integrity, The Exam I Am Handing in Is Solely My OwnDocument11 pagesHonor Code: by The Definition of Academic Integrity, The Exam I Am Handing in Is Solely My OwnHiu Skylar YanNo ratings yet
- (15 Points) Predict The Products of The Following Reactions. Show Relative Stereochemistry (Only One Stereoisomer) Where AppropriateDocument6 pages(15 Points) Predict The Products of The Following Reactions. Show Relative Stereochemistry (Only One Stereoisomer) Where AppropriateparnaNo ratings yet
- Chapter 24 ProblemsDocument13 pagesChapter 24 Problemslynette-wuNo ratings yet
- Practice Exam 2.2-1Document7 pagesPractice Exam 2.2-1jamalNo ratings yet
- Exam 3 PracticeDocument6 pagesExam 3 PracticeVibhav SinghNo ratings yet
- 2423 e 3Document21 pages2423 e 3Abdel Rahman MohamedNo ratings yet
- Chemistry Oo Kashqeysan Imtixaanka Dowlada 2022Document6 pagesChemistry Oo Kashqeysan Imtixaanka Dowlada 2022cazmi AndirahmanNo ratings yet
- Organic Chemistry Past Exam KeyDocument102 pagesOrganic Chemistry Past Exam KeyParker McColl86% (7)
- Chem 114Document12 pagesChem 114lesliemarie272No ratings yet
- Organic Chemistry Questions 3Document12 pagesOrganic Chemistry Questions 3Ram KrishnaNo ratings yet
- CHM207 TutorialDocument3 pagesCHM207 Tutorialit's miaNo ratings yet
- 118A Practice+3Document12 pages118A Practice+3victoryb0% (1)
- Sarp Arca 10 V KimyaDocument2 pagesSarp Arca 10 V KimyanapimyatNo ratings yet
- OCW Exam 1Document10 pagesOCW Exam 1iliketospam123No ratings yet
- Organic Ps Chapter 7Document33 pagesOrganic Ps Chapter 7Mond DamascoNo ratings yet
- TEST 1 CHEM 102 2022 MemoDocument14 pagesTEST 1 CHEM 102 2022 MemoMpho TsheoleNo ratings yet
- CH CH CCH C CHDocument15 pagesCH CH CCH C CHVirgilio Ebajo Jr.No ratings yet
- CHM556 Assignment1Document4 pagesCHM556 Assignment1Saidin AhmadNo ratings yet
- Do Not Open This Test Until Everyone Has OneDocument12 pagesDo Not Open This Test Until Everyone Has OneChemist MeNo ratings yet
- NEPHAR 109 Practice Problems - 2 - G1&G2-1Document3 pagesNEPHAR 109 Practice Problems - 2 - G1&G2-1Amirabbas SaffariNo ratings yet
- Confidential : (Malacca High School Estd.1826)Document9 pagesConfidential : (Malacca High School Estd.1826)ThilagaNo ratings yet
- Practice Exam 2.4Document6 pagesPractice Exam 2.4jamalNo ratings yet
- XI Mid Term QPDocument3 pagesXI Mid Term QPtechnical SiteNo ratings yet
- CHM 241-Practice Exam FinalDocument12 pagesCHM 241-Practice Exam FinalPreeti SharmaNo ratings yet
- Final Exam ReviewDocument7 pagesFinal Exam ReviewMicrorobotRBLXNo ratings yet
- Ch1b Ps3 Key SerDocument7 pagesCh1b Ps3 Key SerRichard ZhuNo ratings yet
- F09 Thermodynamics Exam VA SolutionsDocument9 pagesF09 Thermodynamics Exam VA SolutionsEkimNo ratings yet
- 2021 Princeton Chemistry Lab ExamDocument8 pages2021 Princeton Chemistry Lab ExamJuliet FangNo ratings yet
- Final Key f07Document11 pagesFinal Key f07UpandawayNo ratings yet
- 432georgia Spring 2018 Practice MT 2 - REVISEDDocument14 pages432georgia Spring 2018 Practice MT 2 - REVISEDChemist MeNo ratings yet
- ExamDocument13 pagesExamHamed AliNo ratings yet
- List Number: 1 2 3 4 5 6 7 TotalDocument5 pagesList Number: 1 2 3 4 5 6 7 Totaltcrupi1No ratings yet
- ChemistryDocument34 pagesChemistryIvan Hoo Chean Yieng0% (1)
- Mid TermDocument12 pagesMid TermKaran PrabaNo ratings yet
- H2 Chem Promo 2011Document18 pagesH2 Chem Promo 2011Andrew Seow100% (1)
- 2019-20 DPL1 HW3 v1 PDFDocument3 pages2019-20 DPL1 HW3 v1 PDFWang Hei WongNo ratings yet
- Exam 1 2004Document3 pagesExam 1 2004nanabanana11100% (1)
- SP 2003 Final Organic II 200pts (Weighted As 300)Document23 pagesSP 2003 Final Organic II 200pts (Weighted As 300)Ummi Khairani UrfaNo ratings yet
- IIT JEE Advanced Sample Question Paper With Detailed Solutions 2Document35 pagesIIT JEE Advanced Sample Question Paper With Detailed Solutions 2Manish PilaniaNo ratings yet
- OCHEM Practice FinalsDocument13 pagesOCHEM Practice FinalsNoleNo ratings yet
- Practice ProblemsDocument11 pagesPractice Problemspatricia_moran_4No ratings yet
- Exam 26030 F18Document10 pagesExam 26030 F18Christian CederhornNo ratings yet
- 332 Webexam 4Document7 pages332 Webexam 4Levite DeliveranceNo ratings yet
- Practice Chapter 18 Carboxylic AcidsDocument0 pagesPractice Chapter 18 Carboxylic AcidsRochelle BartiletNo ratings yet
- Chemistry s5 Theory and Pract.Document29 pagesChemistry s5 Theory and Pract.ngabonzizayusuf9No ratings yet
- Chem1701 Gceai Fall 2008 MTDocument5 pagesChem1701 Gceai Fall 2008 MTIbrahim Al-HammadiNo ratings yet
- Sydney Tech 2016 4U Trials & Solutions PDFDocument17 pagesSydney Tech 2016 4U Trials & Solutions PDFSumNo ratings yet
- Gauge Theories Lecture Notes by Matthew de AngelisDocument84 pagesGauge Theories Lecture Notes by Matthew de Angeliscifarha venant100% (1)
- NewDocument7 pagesNewmohibharNo ratings yet
- Coordination Compound Theory - EDocument34 pagesCoordination Compound Theory - Ethinkiit50% (2)
- Tdcocern Emmanouel Tsesmelis EngDocument1 pageTdcocern Emmanouel Tsesmelis Engapi-457316550No ratings yet
- General Navigation Exam 1Document96 pagesGeneral Navigation Exam 1momanbh83% (6)
- Refraction and Reflection of Ultrasonic Waves at Interfaces 5.1 Application of Snell's Law in Ultrasonic TestingDocument25 pagesRefraction and Reflection of Ultrasonic Waves at Interfaces 5.1 Application of Snell's Law in Ultrasonic Testingknizam1971No ratings yet
- Lab 5 Newtons Second LawDocument6 pagesLab 5 Newtons Second LawAndrew GomezNo ratings yet
- Thermodynamics ReviewerDocument8 pagesThermodynamics ReviewerLoala SMDNo ratings yet
- D Block Notes Part - 1Document44 pagesD Block Notes Part - 1Altaf Hussain KhanNo ratings yet
- Draft SurveysDocument109 pagesDraft Surveyslostnfnd100% (2)
- PhilosophyofMind 10007744Document433 pagesPhilosophyofMind 10007744maneeshkNo ratings yet
- Algorithmic Method of Design and Analysis of Fractional Slot Windinf of AC Machine CO-UV-0000313 - 01Document8 pagesAlgorithmic Method of Design and Analysis of Fractional Slot Windinf of AC Machine CO-UV-0000313 - 01Adan SolanoNo ratings yet
- Mod-3. Spur GearDocument18 pagesMod-3. Spur GearSharthak GhoshNo ratings yet
- Homework As PDF-fileDocument5 pagesHomework As PDF-fileyu1rtNo ratings yet
- Rolling Contact Phenomena - Kalker PDFDocument84 pagesRolling Contact Phenomena - Kalker PDFAnonymous eCD5ZRNo ratings yet
- TOS-G8 FORCE MOTION AND ENERGY (1st Grading) .Docx Version 1Document6 pagesTOS-G8 FORCE MOTION AND ENERGY (1st Grading) .Docx Version 1Kimberly Sumbillo MonticalboNo ratings yet
- T220 Testing HandsheetsDocument6 pagesT220 Testing HandsheetsMark VicsonNo ratings yet
- X-Ray Diffraction PrinciplesDocument123 pagesX-Ray Diffraction Principlesatswalla100% (1)
- Blasius PDFDocument2 pagesBlasius PDFvtn_severNo ratings yet
- Frequency Response Cylinder - EnglishDocument7 pagesFrequency Response Cylinder - Englishback1949No ratings yet
- Chapter 2A - Radiation InteractionDocument28 pagesChapter 2A - Radiation InteractionZidan HoumaniNo ratings yet
- SPM 1449 2006 Mathematics p2 BerjawapanDocument18 pagesSPM 1449 2006 Mathematics p2 Berjawapanpss smk selandar71% (7)
- ExperimentDocument2 pagesExperimentSarah Jean TraballoNo ratings yet
- HtKe (1e) PDFDocument120 pagesHtKe (1e) PDFRajat KaliaNo ratings yet
- N - 1431 - PHY Practical2Document7 pagesN - 1431 - PHY Practical2Swarup DevdeNo ratings yet
- Hood Types: Enclosing Hoods Are Those in Which The Source Is Either Partially or Totally Enclosed To Provide TheDocument11 pagesHood Types: Enclosing Hoods Are Those in Which The Source Is Either Partially or Totally Enclosed To Provide TheSudhakar KarnanNo ratings yet
- UIL Science Studying PackDocument89 pagesUIL Science Studying PackJohnNo ratings yet
- Velocity Coefficients For Free Jets From Sharp-Edged OrificesDocument5 pagesVelocity Coefficients For Free Jets From Sharp-Edged OrificesMarco Batista XandóNo ratings yet
- PhysicsBowl 2010 Solutions PDFDocument6 pagesPhysicsBowl 2010 Solutions PDFBryce WongNo ratings yet