Professional Documents
Culture Documents
A Phase Is A Homogenous Portion of The System That Has Uniform Physical and Chemical Characteristics
Uploaded by
Bachir El FilOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A Phase Is A Homogenous Portion of The System That Has Uniform Physical and Chemical Characteristics
Uploaded by
Bachir El FilCopyright:
Available Formats
1. A phase is a homogenous portion of the system that has uniform physical and chemical characteristics. 2.
The 3 micro structural characteristics for multiphase alloy: a. The number of phases present b. The relative portion of phases c. The manner in which the phases are arranged 3. Factors affecting microstructure of an alloy: a. What alloying elements are present b. The concentration of these alloying elements c. The heat treatment of the alloy 4. The system is considered at equilibrium in its most stable state that is its phase characteristics do not change over time. Thermodynamically, the condition for phase equilibrium is that the free energy of the system is a minimum for some sets of combination of temperature, pressure, and composition. 5. Metal stable systems are non equilibrium ones that persist indefinitely and experience imperceptible change with time. 6.
Eutectic
Isomorphous 7. Micro constituents are the microscopic metals or alloys the form after change of phase. Example pearlite and proeutectoid ferrite. 8. Terminal solid solutions are the solid that over composition ranges near the concentration extremities of the phase diagram ( alpha or beta) . Intermediate solid solutions (or intermediate phases) may be found at other than the two composition extremes. May at first appear formidable because there are some invariant points and reactions similar to the eutectic. discrete intermediate compounds rather than solid solutions may be found on the phase diagram, and these compounds have distinct chemical formulas; for metalmetal systems, they are called intermetallic compounds. 9. The Gibbs phase rule is P+F=C+N where P is the number of phases present F is the number of degree of freedom (externally controlled values) C is number of components in the system N is the number of non compositional variables 10. -iron is BCC and austenite is FCC 11. Iron (<0.008 wt%C) and steels (0.008 wt%C to 2.14 wt%C) and Cast Iron ( >2.14 wt%C)
12. Pearlite is made of The layers are the ferrite phase, and the cementite phase 13. (a) A hypoeutectoid alloy n an alloy system exhibiting a eutectoid, any alloy whose composition has an excess of base metal compared with the eutectoid composition, and whose equilibrium microstructure contains some eutectoid structure. Hypereutectoid systems exist below the eutectoid temperature. A hypereutectoid: In an alloy system exhibiting a eutectoid, any alloy whose composition has an excess of alloying element compared with the eutectoid composition, and whose equilibrium microstructure contains some eutectoid structure. (b) The proeutectoid phase is pearlite and (+Fe3C)
(c)
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- SP 1275Document191 pagesSP 1275Ibrahim Naguib0% (1)
- Units and Conversion FactorsDocument30 pagesUnits and Conversion FactorsBun YaminNo ratings yet
- 220/33Kv Gis Substation For Township Limited, Jhajjar, HaryanaDocument655 pages220/33Kv Gis Substation For Township Limited, Jhajjar, HaryanaAman chhabraNo ratings yet
- R600a R1150Document4 pagesR600a R1150elpancasero77No ratings yet
- Design: of Shell & Tube Heat ExchangerDocument27 pagesDesign: of Shell & Tube Heat ExchangerBalamurugan SakthivelNo ratings yet
- A Seminar Report On Self Sustainable BuildingDocument32 pagesA Seminar Report On Self Sustainable BuildingashoknrNo ratings yet
- Soil NailingDocument10 pagesSoil NailingSamirsinh ParmarNo ratings yet
- Teaching The Design of ThermalDocument10 pagesTeaching The Design of ThermalBachir El FilNo ratings yet
- C1 PDFDocument32 pagesC1 PDFBachir El FilNo ratings yet
- 2Document10 pages2Bachir El FilNo ratings yet
- 20Document10 pages20Bachir El FilNo ratings yet
- Non-Vapor Compression HVAC ReportDocument24 pagesNon-Vapor Compression HVAC ReportBachir El FilNo ratings yet
- Non-Vapor Compression HVAC Report PDFDocument199 pagesNon-Vapor Compression HVAC Report PDFBachir El FilNo ratings yet
- Adsorption From Theory To PracticeDocument90 pagesAdsorption From Theory To PracticeManusher JonnoNo ratings yet
- Lecture 11 - Boundary LayersDocument39 pagesLecture 11 - Boundary Layerssaeed_azshNo ratings yet
- Chemical EquilibriumDocument5 pagesChemical EquilibriumBachir El FilNo ratings yet
- Dynamics ExamDocument3 pagesDynamics ExamBachir El FilNo ratings yet
- 3Document7 pages3Bachir El FilNo ratings yet
- AE 3030 Lecture 8Document14 pagesAE 3030 Lecture 8Bachir El FilNo ratings yet
- Battery Cooling REPORT V 03 DMDocument37 pagesBattery Cooling REPORT V 03 DMBachir El FilNo ratings yet
- Sheet of Quals GTDocument1 pageSheet of Quals GTBachir El FilNo ratings yet
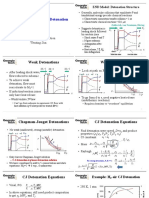
- Planar Detonations and Detonation Structure: Weak Detonations Weak DetonationsDocument4 pagesPlanar Detonations and Detonation Structure: Weak Detonations Weak DetonationsBachir El FilNo ratings yet
- Notes 13 DetonationsDocument13 pagesNotes 13 DetonationsBachir El FilNo ratings yet
- 2 DjetDocument6 pages2 DjetNirmal JayanthNo ratings yet
- Ece 3710Document6 pagesEce 3710Bachir El FilNo ratings yet
- Determining Thermodynamic States: Introduction and OverviewDocument6 pagesDetermining Thermodynamic States: Introduction and OverviewBachir El FilNo ratings yet
- Correction in Mass TransferDocument6 pagesCorrection in Mass TransferBachir El FilNo ratings yet
- 09 KolmDocument44 pages09 Kolmakiakis800No ratings yet
- Mock Oral ExamDocument1 pageMock Oral ExamBachir El FilNo ratings yet
- Lecture 4: Circulation and Vorticity Lecture 4: Circulation and VorticityDocument33 pagesLecture 4: Circulation and Vorticity Lecture 4: Circulation and VorticityPrince Israel EboigbeNo ratings yet
- DateDocument4 pagesDateBachir El FilNo ratings yet
- Notes On Mohr S Circles and Shear StrengthDocument18 pagesNotes On Mohr S Circles and Shear StrengthBachir El FilNo ratings yet
- Heat Transfer Mock Oral ExamDocument1 pageHeat Transfer Mock Oral ExamBachir El FilNo ratings yet
- Shear Strength LectureDocument79 pagesShear Strength LecturenvnrevNo ratings yet
- MECH512 Internal Combustion Engines - Spring 2008 Alan Shihadeh,, RGB 414Document1 pageMECH512 Internal Combustion Engines - Spring 2008 Alan Shihadeh,, RGB 414Bachir El FilNo ratings yet
- Repeatability and Reproducibility PDFDocument13 pagesRepeatability and Reproducibility PDFMJoanaDuarteNo ratings yet
- GFRP BrochureDocument4 pagesGFRP BrochureAnonymous RgjuCNLNo ratings yet
- Manual Midea Ac MPN1 08CR.10CR EN Version1 PDFDocument24 pagesManual Midea Ac MPN1 08CR.10CR EN Version1 PDFwayne dinhNo ratings yet
- Natural Fiber Composite Design and Characterization For Limit Stress Prediction in Multiaxial Stress StateDocument14 pagesNatural Fiber Composite Design and Characterization For Limit Stress Prediction in Multiaxial Stress Statechristian emeka okaforNo ratings yet
- Difference Between RCD and RCBODocument2 pagesDifference Between RCD and RCBOronnelNo ratings yet
- Model Estimates TubewellDocument137 pagesModel Estimates TubewellBilal A Barbhuiya0% (1)
- Assignment 2 SolutionDocument22 pagesAssignment 2 SolutionAshlyn CicilyNo ratings yet
- Howo A7 Cam Nang Sua Chua Dien00028Document1 pageHowo A7 Cam Nang Sua Chua Dien00028manhNo ratings yet
- CE PROJECT 1 - 1st Semester SY 2019-2020Document1 pageCE PROJECT 1 - 1st Semester SY 2019-2020Jose Mari RoldanNo ratings yet
- WDE - Anganwadi Building - PasthalaDocument52 pagesWDE - Anganwadi Building - PasthalaGurram Lakshmi NavakanthNo ratings yet
- Career Objective:: Ricky Solis SibalDocument4 pagesCareer Objective:: Ricky Solis Sibalroselyn sibalNo ratings yet
- SECTION 15920 Pneumatic Controls Rev 0Document39 pagesSECTION 15920 Pneumatic Controls Rev 0Munir RasheedNo ratings yet
- History of PiezopolymersDocument14 pagesHistory of PiezopolymersrachmajuwitaNo ratings yet
- Example Design The Reinforced Concrete Slab For The Building Shown in Fig. If L.L. 3 / and Add. D.L. 2 /, FC' 30mpa, Fy 420mpaDocument5 pagesExample Design The Reinforced Concrete Slab For The Building Shown in Fig. If L.L. 3 / and Add. D.L. 2 /, FC' 30mpa, Fy 420mpaريام الموسويNo ratings yet
- Minutes of The Meeting: AttendeesDocument3 pagesMinutes of The Meeting: AttendeesADEN LIUNo ratings yet
- Design of Bolted Flange Connections - Status of EN Standards: July 2020Document28 pagesDesign of Bolted Flange Connections - Status of EN Standards: July 2020Dan PastorNo ratings yet
- Expona Control Product SpecDocument1 pageExpona Control Product SpecFloorkitNo ratings yet
- Retrofitting Estimate ReportDocument8 pagesRetrofitting Estimate ReportPsiko QuattroNo ratings yet
- JMS PIPING REPLACEMENT IWR BT1 2019 023 (UTY) Rev01Document21 pagesJMS PIPING REPLACEMENT IWR BT1 2019 023 (UTY) Rev01Mohd FarHan Alias100% (1)
- TPV Seal Geometry Effects On Compression Set (CSet) and Compression Load Defglection - Above-The-belt 2006Document20 pagesTPV Seal Geometry Effects On Compression Set (CSet) and Compression Load Defglection - Above-The-belt 2006KAMAL BEHLNo ratings yet
- Eng TipsDocument5 pagesEng TipsBenedictus MurdonoNo ratings yet
- Austempered Ductile Iron Castings: Standard Specification ForDocument8 pagesAustempered Ductile Iron Castings: Standard Specification ForJosé Ramón GutierrezNo ratings yet
- Detail Engineering Services For Engen Tank X368 RebuildDocument9 pagesDetail Engineering Services For Engen Tank X368 RebuildpavanNo ratings yet
- Effect of Welding Current On The Mechanical and StructuralDocument8 pagesEffect of Welding Current On The Mechanical and StructuralBhramandhikaNalendraGhuptaNo ratings yet
- Declaration of Performance Hilti SF DoP 012 EN Declaration of Performance IBD WWI 00000000000002988797 000Document1 pageDeclaration of Performance Hilti SF DoP 012 EN Declaration of Performance IBD WWI 00000000000002988797 000Kovacs Zsolt-IstvanNo ratings yet