Professional Documents
Culture Documents
Coagulants Kimal
Uploaded by
Kimal WasalathilakeOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Coagulants Kimal
Uploaded by
Kimal WasalathilakeCopyright:
Available Formats
DISCUSSION
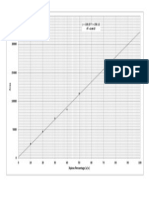
Coagulants and Functions Finely dispersed solids (colloids) suspended in wastewater are stabilized by negative electric charges on their surfaces, causing them to repel each other. Since this prevents these charged particles from colliding to form larger masses, called flocs, they do not settle. To assist in the removal of colloidal particles from suspension, chemical coagulation is required. Coagulants neutralize the repulsive electrical charges (typically negative) surrounding particles allowing them to "stick together" creating clumps or flocs. Commonly used metal coagulants can be categorized into two groups as Aluminium based coagulants and Iron based coagulants. Aluminium sulfate, Aluminium chloride and Sodium aluminate are aluminium based coagulants. Ferric sulfate, Ferrous sulfate, Ferric chloride and Ferric chloride sulfate are Iron based coagulants. Apart from that, coagulants can also be categorized as organic and inorganic coagulants. How to find the optimum dosage of coagulant for a given sample Many variables affect the destabilization process of the agglomerated particles. The efficiency of the coagulation heavily depends on the Ph value of the solution and the dosage of the coagulant used. It is important to find the optimum dosage of the coagulant which should be used because care must be taken not to overdose the coagulants as this can cause a complete charge reversal and re-stabilize the colloid complex. In this experiment jar test was used to find the optimum coagulant dosage for the waste water sample. Four 450 ml waste water samples were prepared in beakers. In order to find the effect of one variable the other variable should be kept in a constant value. Another jar test was done earlier by the other group to find the optimum Ph range and the optimum ph was observed to be between 9 and 10.So the ph value of those 4 samples were brought to the range of 9-10 by adding NaOH. Four different samples of coagulant was prepared as follows, Sample 1 : 10 ml of 3% alum ( 0.3g in 10 ml of distilled water) Sample 2 : 10 ml of 4% alum (0.4g in 10 ml of distilled water) Sample 3 : 10 ml of 5% alum (0.5g in 10 ml of distilled water) Sample 4 : 10 ml of 6% alum (0.6g in 10 ml of distilled water) Samples were added to each of the waste water samples and were initially stirred at 100rpm for 1 minute to achieve good mixing of coagulants. Then they were stirred at 40rpm for 20-30 minutes to form flocs. After that they were kept again for about 30 minutes to settle.
Supernatant solution was taken from each beaker and the turbidity was measured. A graph was plotted on Turbity vs. Coagulant dosage. The dosage which gives the minimum turbidity was considered as the optimum coagulant dosage for the waste water sample.
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Financing The New VentureDocument46 pagesFinancing The New VentureAnisha HarshNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Lesson 6 Intercultural ComDocument25 pagesLesson 6 Intercultural ComBrave MitraNo ratings yet
- Construction Quality Management 47 PDFDocument47 pagesConstruction Quality Management 47 PDFCarl WilliamsNo ratings yet
- 3 - QMT425-T3 Linear Programming (29-74)Document46 pages3 - QMT425-T3 Linear Programming (29-74)Ashraf RadzaliNo ratings yet
- Project Report On Reliance TrendsDocument91 pagesProject Report On Reliance TrendsSubash Tej T50% (4)
- Introduction To The Philosophy of The Human PersonDocument21 pagesIntroduction To The Philosophy of The Human Personanon_254928515No ratings yet
- A Beginner's Guide To Reading Jung - Jungian Center For The Spiritual SciencesDocument6 pagesA Beginner's Guide To Reading Jung - Jungian Center For The Spiritual SciencesRosa ChacónNo ratings yet
- Distillation Tutorial in HYSYSDocument7 pagesDistillation Tutorial in HYSYScmlim100% (2)
- Influent, C Effluent, C V (M) : Ai A AoDocument7 pagesInfluent, C Effluent, C V (M) : Ai A AoAnkit BansalNo ratings yet
- 0708 Conduction Convection RadiationDocument23 pages0708 Conduction Convection RadiationBella LimNo ratings yet
- Chemical Reactors: DC DT RDocument8 pagesChemical Reactors: DC DT ROsas Jessica UwoghirenNo ratings yet
- Moisture LabDocument5 pagesMoisture LabKimal WasalathilakeNo ratings yet
- Mass BalanceDocument17 pagesMass BalanceKimal WasalathilakeNo ratings yet
- Reduction of Suspended SolidsDocument3 pagesReduction of Suspended SolidsKimal WasalathilakeNo ratings yet
- SaponificationDocument1 pageSaponificationKimal WasalathilakeNo ratings yet
- Chapter9 Thermal Performance Dec09Document20 pagesChapter9 Thermal Performance Dec09Animasahun Olamide HammedNo ratings yet
- IPA+IPA+Xylene Calibration Curvexylene Calibration CurveDocument1 pageIPA+IPA+Xylene Calibration Curvexylene Calibration CurveKimal WasalathilakeNo ratings yet
- Chemistry of Ethylene Production From NaphthaDocument2 pagesChemistry of Ethylene Production From NaphthaKimal Wasalathilake0% (1)
- Test PresentationDocument1 pageTest PresentationKimal WasalathilakeNo ratings yet
- Process Selection Catalytic CrackingDocument5 pagesProcess Selection Catalytic CrackingKimal WasalathilakeNo ratings yet
- TeletubbiesDocument2 pagesTeletubbiesKimal WasalathilakeNo ratings yet
- Feasibility Report of Producing Renewable Energy in HouseholdDocument12 pagesFeasibility Report of Producing Renewable Energy in HouseholdKimal WasalathilakeNo ratings yet
- Imad Eddin Jamali: P.O. Box 4686 AJMAN, U.A.E. Tel: 050 - 5973166Document4 pagesImad Eddin Jamali: P.O. Box 4686 AJMAN, U.A.E. Tel: 050 - 5973166imadjamali100% (1)
- Question Bank of Financial Management - 2markDocument16 pagesQuestion Bank of Financial Management - 2marklakkuMSNo ratings yet
- Cohort 2 Assignment Essay FinalDocument4 pagesCohort 2 Assignment Essay Finalapi-652640066No ratings yet
- Topic Categorization Based On User Behaviour in Random Social Networks Using Firefly AlgorithmDocument5 pagesTopic Categorization Based On User Behaviour in Random Social Networks Using Firefly AlgorithmBONFRINGNo ratings yet
- Oils and Lard by Fourier Transform Infrared Spectroscopy. Relationships Between Composition and Frequency of Concrete Bands in The Fingerprint Region.Document6 pagesOils and Lard by Fourier Transform Infrared Spectroscopy. Relationships Between Composition and Frequency of Concrete Bands in The Fingerprint Region.Nong NakaNo ratings yet
- Hemzo M. Marketing Luxury Services. Concepts, Strategy and Practice 2023Document219 pagesHemzo M. Marketing Luxury Services. Concepts, Strategy and Practice 2023ichigosonix66No ratings yet
- Two Steps From Hell Full Track ListDocument13 pagesTwo Steps From Hell Full Track ListneijeskiNo ratings yet
- 07 Indian WeddingDocument2 pages07 Indian WeddingNailah Al-FarafishahNo ratings yet
- ViShNu-Virachita Rudra StotramDocument6 pagesViShNu-Virachita Rudra StotramBhadraKaaliNo ratings yet
- Psychological Science1Document23 pagesPsychological Science1Jatin RaghavNo ratings yet
- 5.1 ReteachDocument2 pages5.1 ReteachCarlos Pastrana0% (1)
- THE STUDY OF THE TESTIMONIES. - No. 1. A Series Presented at The 1893 General Conference Session by ELDER J. N. LOUGHBOROUGHDocument16 pagesTHE STUDY OF THE TESTIMONIES. - No. 1. A Series Presented at The 1893 General Conference Session by ELDER J. N. LOUGHBOROUGHKunedog1No ratings yet
- Hydrostatic PressureDocument13 pagesHydrostatic Pressureapi-2859151810% (1)
- NO KEY Synonyms-And-Antonyms-Describing-Character-Grammar-Drills-Wordsearches - 81561Document1 pageNO KEY Synonyms-And-Antonyms-Describing-Character-Grammar-Drills-Wordsearches - 81561Helena MariñoNo ratings yet
- The Discovery of The Tun Huang Library and Its Effect On Chinese StudiesDocument21 pagesThe Discovery of The Tun Huang Library and Its Effect On Chinese Studiesfabricatore_21639575No ratings yet
- Week 006 - Module Sample Works of Well-Known WritersDocument13 pagesWeek 006 - Module Sample Works of Well-Known WritersLeona April DarriguezNo ratings yet
- Nift B.ftech Gat PaperDocument7 pagesNift B.ftech Gat PapergoelNo ratings yet
- Chemical Engineering Science: N. Ratkovich, P.R. Berube, I. NopensDocument15 pagesChemical Engineering Science: N. Ratkovich, P.R. Berube, I. Nopensvasumalhotra2001No ratings yet
- International Accounting Standard 36 (IAS 36), Impairment of AssetsDocument10 pagesInternational Accounting Standard 36 (IAS 36), Impairment of AssetsMadina SabayevaNo ratings yet
- Resume Design 2019Document2 pagesResume Design 2019ezke4pq2100% (2)
- Meditation Club of RUET Asif EEE 17 PDFDocument2 pagesMeditation Club of RUET Asif EEE 17 PDFShovonNo ratings yet
- TIFR Pamphlet On Homological MethodsDocument105 pagesTIFR Pamphlet On Homological MethodsRAMJANNo ratings yet
- 308 MSR Folktale Sir CedricDocument3 pages308 MSR Folktale Sir Cedricapi-242415607No ratings yet