Professional Documents
Culture Documents
Pollution of Ocean Environment
Uploaded by
roy thomasOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pollution of Ocean Environment
Uploaded by
roy thomasCopyright:
Available Formats
Environmental Management System An environmental management system is the process used by an organization to man age, review, correct, and
improve the organization s approach to business. Employe es are asked to consider how they affect the environment every day. An EMS offe rs a structured way to incorporate environmental considerations into day-to-day operations; it promotes continual improvement of the environment and human healt h. --------------------------------------------------------------------------------------------------------------Photosynthesis -[photo-], "light," and s???es?? [synthesis], "putting together", "composition") is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight.[1] Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photo synthetic organisms are called photoautotrophs, since they can create their own food. In plants, algae, and cyanobacteria, photosynthesis uses carbon dioxide an d water, releasing oxygen as a waste product. Photosynthesis is vital for all ae robic life on Earth. In addition to maintaining normal levels of oxygen in the a tmosphere, photosynthesis is the source of energy for nearly all life on earth, either directly, through primary production, or indirectly, as the ultimate sour ce of the energy in their food,[2] the exceptions being chemoautotrophs that liv e in rocks or around deep sea hydrothermal vents. The rate of energy capture by photosynthesis is immense, approximately 100 terawatts,[3] which is about six ti mes larger than the power consumption of human civilization.[4] As well as energ y, photosynthesis is also the source of the carbon in all the organic compounds within organisms' bodies. In all, photosynthetic organisms convert around 100 115 petagrams of carbon into biomass per year.[5][6] Factors The leaf is the primary site of photosynthesis in plants. There are three main factors affecting photosynthesis and several corollary fact ors. The three main are: Light irradiance and wavelength Carbon dioxide concentration Temperature. Light intensity (irradiance), wavelength and temperature In the early 20th century, Frederick Frost Blackman along with Albert Einstein i nvestigated the effects of light intensity (irradiance) and temperature on the r ate of carbon assimilation. At constant temperature, the rate of carbon assimilation varies with irradia nce, initially increasing as the irradiance increases. However, at higher irradi ance, this relationship no longer holds and the rate of carbon assimilation reac hes a plateau. (Irradiance is the power of electromagnetic radiation per unit area (radiative f lux) incident on a surface. Radiant emittance or radiant exitance is the power p er unit area radiated by a surface. The SI units for all of these quantities are watts per square meter (W/m2), while the cgs units are ergs per square centimet er per second (ergcm-2s-1, often used in astronomy). These quantities are sometime s called intensity, but this usage leads to confusion with radiant intensity, wh ich has different units. All of these quantities characterize the total amount of radiation present, at a ll frequencies. It is also common to consider each frequency in the spectrum sep
arately. When this is done for radiation incident on a surface, it is called spe ctral irradiance, and has SI units W/m3, or commonly Wm-2nm-1. If a point source radiates light uniformly in all directions through a non-absor ptive medium, then the irradiance decreases in proportion to the square of the d istance from the object.) At constant irradiance, the rate of carbon assimilation increases as the tem perature is increased over a limited range. This effect is seen only at high irr adiance levels. At low irradiance, increasing the temperature has little influen ce on the rate of carbon assimilation. These two experiments illustrate vital points: First, from research it is known that, in general, photochemical reactions are not affected by temperature. Howev er, these experiments clearly show that temperature affects the rate of carbon a ssimilation, so there must be two sets of reactions in the full process of carbo n assimilation. These are, of course, the light-dependent 'photochemical' stage and the light-independent, temperature-dependent stage. Second, Blackman's exper iments illustrate the concept of limiting factors. Another limiting factor is th e wavelength of light. Cyanobacteria, which reside several meters underwater, ca nnot receive the correct wavelengths required to cause photoinduced charge separ ation in conventional photosynthetic pigments. To combat this problem, a series of proteins with different pigments surround the reaction center. This unit is c alled a phycobilisome. Carbon dioxide levels and photorespiration As carbon dioxide concentrations rise, the rate at which sugars are made by the light-independent reactions increases until limited by other factors. RuBisCO, t he enzyme that captures carbon dioxide in the light-independent reactions, has a binding affinity for both carbon dioxide and oxygen. When the concentration of carbon dioxide is high, RuBisCO will fix carbon dioxide. However, if the carbon dioxide concentration is low, RuBisCO will bind oxygen instead of carbon dioxide . This process, called photorespiration, uses energy, but does not produce sugar s. RuBisCO oxygenase activity is disadvantageous to plants for several reasons: One product of oxygenase activity is phosphoglycolate (2 carbon) instead of 3-phosphoglycerate (3 carbon). Phosphoglycolate cannot be metabolized by the Cal vin-Benson cycle and represents carbon lost from the cycle. A high oxygenase act ivity, therefore, drains the sugars that are required to recycle ribulose 5-bisp hosphate and for the continuation of the Calvin-Benson cycle. Phosphoglycolate is quickly metabolized to glycolate that is toxic to a plan t at a high concentration; it inhibits photosynthesis. Salvaging glycolate is an energetically expensive process that uses the glyc olate pathway, and only 75% of the carbon is returned to the Calvin-Benson cycle as 3-phosphoglycerate. The reactions also produce ammonia (NH3), which is able to diffuse out of the plant, leading to a loss of nitrogen. A highly simplified summary is: 2 glycolate + ATP ? 3-phosphoglycerate + carbon dioxide + ADP + NH3 The salvaging pathway for the products of RuBisCO oxygenase activity is more com monly known as photorespiration, since it is characterized by light-dependent ox ygen consumption and the release of carbon dioxide. --------------------------------------------------------------------------------------------------------------Hypoxia (environmental)
Hypoxia, or oxygen depletion, is a phenomenon that occurs in aquatic environment s as dissolved oxygen (DO; molecular oxygen dissolved in the water) becomes redu ced in concentration to a point where it becomes detrimental to aquatic organism s living in the system. Dissolved oxygen is typically expressed as a percentage of the oxygen that would dissolve in the water at the prevailing temperature and salinity (both of which affect the solubility of oxygen in water; see oxygen sa turation and underwater). An aquatic system lacking dissolved oxygen (0% saturat ion) is termed anaerobic, reducing, or anoxic; a system with low concentration in the range between 1 and 30% saturation is called hypoxic or dysoxic. Most fish can not live below 30% saturation. A "healthy" aquatic environment should seldom exp erience less than 80%. The exaerobic zone is found at the boundary of anoxic and hypoxic zones. Contents 1 2 3 4 5 6 7 Where hypoxia occurs Causes of hypoxia Solutions Bog chemistry See also References External links
Where hypoxia occurs Hypoxia can occur throughout the water column and also at high altitudes as well as near sediments on the bottom. It usually extends throughout 20-50% of the wa ter column, but depending on the water depth and location of pycnoclines (rapid changes in water density with depth)[1] it can occur in 10-80% of the water colu mn. For example, in a 10-meter water column, it can reach up to 2 meters below t he surface. In a 20-meter water column, it can extend up to 8 meters below the surface.[2] Hypoxia can also occur outside of an aquatic environment, in conditions where th e oxygen content of the air is reduced. This is common, for example, in the seal ed burrows of some subterranean animals, such as blesmols.[3] causes of hypoxia Oxygen depletion can result from a number of natural factors, but is most often a concern as a consequence of pollution and eutrophication in which plant nutrie nts enter a river, lake, or ocean, and phytoplankton blooms are encouraged. Whil e phytoplankton, through photosynthesis, will raise DO saturation during dayligh t hours, the dense population of a bloom reduces DO saturation during the night by respiration. When phytoplankton cells die, they sink towards the bottom and a re decomposed by bacteria, a process that further reduces DO in the water column . If oxygen depletion progresses to hypoxia, fish kills can occur and invertebra tes like worms and clams on The floor is covered with crabs, fish, and clams apparently dead or dying from o xygen depletion. the bottom may be killed as well.
Solutions To combat hypoxia, it is essential to reduce the amount of land-derived nutrient s reaching rivers in runoff. Defensively this can be done by improving sewage tr eatment and by reducing the amount of fertilizers leaching into the rivers. Offe nsively this can be done by restoring natural environments along a river; marshe s are particularly effective in reducing the amount of phosphorus and nitrogen ( nutrients) in water. Technological solutions are also possible, such as that used in the redeveloped Salford Docks area of the Manchester Ship Canal in England, where years of runof f from sewers and roads had accumulated in the slow running waters. In 2001 a co mpressed air injection system was introduced, which raised the oxygen levels in the water by up to 300%. The resulting improvement in water quality led to an in crease in the number of invertebrate species, such as freshwater shrimp, to more than 30. Spawning and growth rates of fish species such as roach and perch also increased to such an extent that they are now amongst the highest in England.[6 ] In a very short time the oxygen saturation can drop to zero when offshore blowin g winds drive surface water out and anoxic depthwater rises up. At the same time a decline in temperature and a rise in salinity is observed (from the longterm ecological observatory in the seas at Kiel Fjord, Germany). New approaches of lo ng-term monitoring of oxygen regime in the ocean observe online the behavior of fish and zooplankton, which changes drastically under reduced oxygen saturations (ecoSCOPE) and already at very low levels of water pollution. --------------------------------------------------------------------------------------------------------------Phytoplankton (English pronunciation: /?fa?to?'pl?kt?n/) are the autotrophic component of the plankton community. The name comes from the Greek words f?t?? (phyton), meaning "plant", and p?a??t?? (planktos), meaning " wanderer" or "drifter".[1] Most phytoplankton are too small to be individually s een with the unaided eye. However, when present in high enough numbers, they may appear as a green discoloration of the water due to the presence of chlorophyll within their cells (although the actual color may vary with the species of phytoplankton present due to varying levels of chlorophyll or the pre sence of accessory pigments such as phycobiliproteins, xanthophylls, etc.). Ecology Phytoplankton are photosynthesizing microscopic organisms that inhabit the upper sunlit layer of almost all oceans and bodies of fresh water. They are agents fo r "primary production," the creation of organic compounds from carbon dioxide di ssolved in the water, a process that sustains the aquatic food web.[2] Phytoplan kton obtain energy through the process of photosynthesis and must therefore live in the well-lit surface layer (termed the euphotic zone) of an ocean, sea, lake , or other body of water. Phytoplankton account for half of all photosynthetic a ctivity on Earth.[3] Thus phytoplankton are responsible for much of the oxygen p resent in the Earth's atmosphere half of the total amount produced by all plant life.[4] Their cumulative energy fixation in carbon compounds (primary Phytoplankton are the foundation of the oc eanic food chain production) is the basis for the vast majority of oceanic and a lso many freshwater food webs (chemosynthesis is a notable exception). The effec ts of anthropogenic warming on the global population of phytoplankton is an area
of active research. Changes in the vertical stratification of the water column, the rate of temperature-dependent biological reactions, and the atmospheric sup ply of nutrients are not expected to have important effects on future phytoplank ton productivity.[5][6] Additionally, changes in the mortality of phytoplankton due to rates of zooplankton grazing may be significant. As a si de note, one of the more remarkable food chains in the ocean remarkable because of the small number of links is that of phytoplankton-feeding krill (a crustacea n similar to a tiny shrimp) feeding baleen whales. Phytoplankton are also crucially dependent on minerals. These are primarily macr onutrients such as nitrate, phosphate or silicic acid, whose availability is gov erned by the balance between the so-called biological pump and upwelling of deep , nutrient-rich waters. However, across large regions of the World Ocean such as the Southern Ocean, phytoplankton are also limited by the lack of the micronutr ient iron. This has led to some scientists advocating iron fertilization as a me ans to counteract the accumulation of human- produced carbon dioxide (CO2) in th e atmosphere.[7] Large-scale experiments have added iron (usually as salts such as iron sulphate) to the oceans to promote phytoplankton growth and draw atmosph eric CO2 into the ocean. However, controversy about manipulating the ecosystem a nd the efficiency of iron fertilization has slowed such experiments.[8] While almost all phytoplankton species are obligate photoautotrophs, there are s ome that are mixotrophic and other, non-pigmented species that are actually heterotrophic (the latter are often viewed as zooplank ton). Of these, the best known are dinoflagellate genera such as Noctiluca and D inophysis, that obtain organic carbon by ingesting other organisms or detrital m aterial. The term phytoplankton encompasses all photoautotrophic microorganisms in aquati c food webs. Phytoplankton serve as the base of the aquatic food web, providing an essential ecological function for all aquatic life. However, unlike terrestri al communities, where most autotrophs are plants, phytoplankton are a diverse gr oup, incorporating protistan eukaryotes and both eubacterial and archaebacterial prokaryotes. There are about 5,000 known species of marine phytoplankton.[9] Th ere is uncertainty in how such diversity has evolved in an environment where com petition for only a few resources would suggest limited potential for niche differentiation.[10] When two currents (in this case the Oyashio and Kuroshio currents) collide, they create eddies. Phytoplankton become concentrated along the boundaries of these eddies, tracing out the motions of the water. Algal bloom off south England In terms of numbers, the most important groups of phytoplankton include the diat oms, cyanobacteria and dinoflagellates, although many other groups of algae are represented. One group, the coccolithophorids, is responsible (in part) for the release of significant amounts of dimethyl sulfide (DMS) into the atmosphere. DM S is converted to sulfate and these sulfate molecules act as cloud condensation nuclei, increasing general cloud cover. In oligotrophic oceanic regions such as the Sargasso Sea or the South Pacific Gyre, phytoplankton is dominated by the sm all sized cells, called picoplankton, mostly composed of cyanobacteria (Prochlor ococcus, Synechococcus) and picoeucaryotes such as Micromonas. Environmental threats
A 2010 study published in Nature found that marine phytoplankton have declined s ubstantially in the world's oceans over the past century. Since 1950 alone, phyt oplankton concentrations in surface waters were reported to have decreased by ab out 40%, possibly in response to ocean warming.[11][12] The study generated debate among scientists and led to several communications, also publis hed in Nature.[13][14] [15][16] This study has not yet been substantiated. Aquaculture Phytoplankton are a key food item in both aquaculture and mariculture. Both util ize phytoplankton as food for the animals being farmed. In mariculture, the phyt oplankton is naturally occurring and is introduced into enclosures with the norm al circulation of seawater. In aquaculture, phytoplankton must be obtained and i ntroduced directly. The plankton can either be collected from a body of water or cultured, though the former method is seldom used. Phytoplankton is used as a foodstock for the produ ction of rotifers,[17] which are in turn used to feed other organisms. Phytoplan kton is also used to feed many varieties of aquacultured molluscs, including pea rl oysters and giant clams. The production of phytoplankton under artificial conditions is itself a form of aquaculture. Phytoplankton is cultured for a variety of purposes, including food stock for other aquacultured organisms,[17] a nutritional supplement for captive invertebrates in aquaria. Culture sizes range from small-scale laboratory cultu res of less than 1L to several tens of thousands of liters for commercial aquacu lture.[17] Regardless of the size of the culture, certain conditions must be pro vided for efficient growth of plankton. The majority of cultured plankton is mar ine, and seawater of a specific gravity of 1.010 to 1.026 may be used as a cultu re medium. This water must be sterilized, usually by either high temperatures in an autoclave or by exposure to ultraviolet radiation, to prevent biological con tamination of the culture. Various fertilizers are added to the culture medium t o facilitate the growth of plankton. A culture must be aerated or agitated in so me way to keep plankton suspended, as well as to provide dissolved carbon dioxid e for photosynthesis. In addition to constant aeration, most cultures are manual ly mixed or stirred on a regular basis. Light must be provided for the growth of phytoplankton. The colour temperature of illumination should be approximately 6 ,500 K, but values from 4,000 K to upwards of 20,000 K have been used successful ly. The duration of light exposure should be approximately 16 hours daily; this is the most efficient artificial day length.[17] -------------------------------------------------------------------------------------------------------------Global warming From Wikipedia, the free encyclopedia This article is about the change in climate Earth is currently experiencing. For general discussion of how Earth's climate can change, see Climate change. Page semi-protected This is a featured article. Click here for more information. ] Global mean land-ocean temperature change from 1880 2011, relative to the 1951 1980 mean. The black line is the annual mean and the red line is the 5-year running m ean. The green bars show uncertainty estimates. Source: NASA GISS The map shows the 10-year average (2000 2009) global mean temperature anomaly rela tive to the 1951 1980 mean. The largest temperature increases are in the Arctic an d the Antarctic Peninsula. Source: NASA Earth Observatory[1] Fossil fuel related CO2 emissions compared to five of IPCC's emissions scenarios . The dips are related to global recessions. Data from IPCC SRES scenarios; Data spreadsheet included with International Energy Agency's "CO2 Emissions from Fue l Combustion 2010 Highlights"; and Supplemental IEA data. Image source: Skeptica l Science
Global warming refers to the current rise in the average temperature of Earth's atmosphere and oceans and its projected continuation. In the last 100 years, Ear th's average surface temperature increased by about 0.8 C (1.4 F) with about two t hirds of the increase occurring over just the last three decades.[2] Warming of the climate system is unequivocal, and scientists are more than 90% certain most of it is caused by increasing concentrations of greenhouse gases produced by hu man activities such as deforestation and burning fossil fuels.[3][4][5][6] These findings are recognized by the national science academies of all the major indu strialized countries.[7][A] Climate model projections are summarized in the 2007 Fourth Assessment Report (A R4) by the Intergovernmental Panel on Climate Change (IPCC). They indicate that during the 21st century the global surface temperature is likely to rise a furth er 1.1 to 2.9 C (2 to 5.2 F) for their lowest emissions scenario and 2.4 to 6.4 C ( 4.3 to 11.5 F) for their highest.[8] The ranges of these estimates arise from the use of models with differing sensitivity to greenhouse gas concentrations.[9][1 0] An increase in global temperature will cause sea levels to rise and will change the amount and pattern of precipitation, and a probable expansion of subtropical deserts.[11] Warming is expected to be strongest in the Arctic and would be ass ociated with continuing retreat of glaciers, permafrost and sea ice. Other likel y effects of the warming include more frequent occurrence of extreme weather eve nts including heat waves, droughts and heavy rainfall events, species extinction s due to shifting temperature regimes, and changes in crop yields. Warming and r elated changes will vary from region to region around the globe, with projection s being more robust in some areas than others.[12] In a 4 C world[clarification n eeded], the limits for human adaptation are likely to be exceeded in many parts of the world, while the limits for adaptation for natural systems would largely be exceeded throughout the world. Hence, the ecosystem services upon which human livelihoods depend would not be preserved.[13] Most countries are parties to the United Nations Framework Convention on Climate Change (UNFCCC),[14] whose ultimate objective is to prevent "dangerous" anthrop ogenic (i.e., human-induced) climate change.[15] Parties to the UNFCCC have adop ted a range of policies designed to reduce greenhouse gas emissions[16]:10[17][1 8][19]:9 and to assist in adaptation to global warming.[16]:13[19]:10[20][21] Pa rties to the UNFCCC have agreed that deep cuts in emissions are required,[22] an d that future global warming should be limited to below 2.0 C (3.6 F) relative to the pre-industrial level.[22][B] 2011 analyses by the United Nations Environment Programme[23] and International Energy Agency[24] suggest that current efforts to reduce emissions may be inadequately stringent to meet the UNFCCC's 2 C target . -------------------------------------------------------------------------------------------------------------A carbon sink is a natural or artificial reservoir that accumulates and stores s ome carbon-containing chemical compound for an indefinite period. The process by which carbon sinks remove carbon dioxide (CO2) from the atmosphere is known as carbon sequestration. Public awareness of the significance of CO2 sinks has grow n since passage of the Kyoto Protocol, which promotes their use as a form of car bon offset. The main natural sinks are: Absorption of carbon dioxide by the oceans via physicochemical and biologica l processes Photosynthesis by terrestrial plants Natural sinks are typically much larger than artificial sinks. The main artifici
al sinks are: Landfills Carbon capture and storage proposals Carbon sources include: Fossil fuels Farmland; there are proposals for improvements in farming practices to rever se this.
Oceans are at present CO2 sinks, and represent the largest active carbon sink on Earth, absorbing more than a quarter of the carbon dioxide that humans put into the air.[12] On longer timescales they may be both sources and sinks - during i ce ages CO2 levels decrease to ~180 ppmv, and much of this is believed to be sto red in the oceans. As ice ages end, CO2 is released from the oceans and CO2 leve ls during previous interglacials have been around ~280 ppmv. This role as a sink for CO2 is driven by two processes, the solubility pump and the biological pump .[13] The former is primarily a function of differential CO2 solubility in seawa ter and the thermohaline circulation, while the latter is the sum of a series of biological processes that transport carbon (in organic and inorganic forms) fro m the surface euphotic zone to the ocean's interior. A small fraction of the org anic carbon transported by the biological pump to the seafloor is buried in anox ic conditions under sediments and ultimately forms fossil fuels such as oil and natural gas. At the present time, approximately one third[14] of human generated emissions ar e estimated to be entering the ocean. The solubility pump is the primary mechani sm driving this, with the biological pump playing a negligible role. This stems from the limitation of the biological pump by ambient light and nutrients requir ed by the phytoplankton that ultimately drive it. Total inorganic carbon is not believed to limit primary production in the oceans, so its increasing availabili ty in the ocean does not directly affect production (the situation on land is di fferent, since enhanced atmospheric levels of CO2 essentially "fertilize" land p lant growth). However, ocean acidification by invading anthropogenic CO2 may aff ect the biological pump by negatively impacting calcifying organisms such as coc colithophores, foraminiferans and pteropods. Climate change may also affect the biological pump in the future by warming and stratifying the surface ocean, thus reducing the supply of limiting nutrients to surface waters. In January 2009, the Monterey Bay Aquarium Research Institute and the National O ceanic and Atmospheric Administration announced a joint study to determine wheth er the ocean off the California coast was serving as a carbon source or a carbon sink. Principal instrumentation for the study will be self-contained CO2 monito rs placed on buoys in the ocean. They will measure the partial pressure of CO2 i n the ocean and the atmosphere just above the water surface.[15] In February 2009, Science Daily reported that the Southern Indian Ocean is becom ing less effective at absorbing carbon dioxide due to changes to the regions cli mate which include higher wind speeds.[16] --------------------------------------------------------------------------------------------------------------Eutrophication (Greek: eutrophia healthy, adequate nutrition, development; German: Eutrophie) or more precisely hypertrophication, is the movement of a body of wa ter's trophic status in the direction of increasing biomass, by the addition of artificial or natural substances, such as nitrates and phosphates, through ferti lizers or sewage, to an aquatic system.[1] In other terms, it is the "bloom" or great increase of phytoplankton in a water body. Negative environmental effects
include hypoxia, the depletion of oxygen in the water, which induces reductions in specific fish and other animal populations. Other species (such as Nomura's j ellyfish in Japanese waters) may experience an increase in population that negat ively affects other species. Eutrophication can be human-caused or natural. Untreated sewage effluent and agr icultural run-off carrying fertilizers are examples of human-caused eutrophicati on. However, it also occurs naturally in situations where nutrients accumulate ( e.g. depositional environments), or where they flow into systems on an ephemeral basis. Eutrophication generally promotes excessive plant growth and decay, favo uring simple algae and plankton over other more complicated plants, and causes a severe reduction in water quality. Phosphorus is a necessary nutrient for plant s to live, and is the limiting factor for plant growth in many freshwater ecosys tems. The addition of phosphorous increases algal growth. These algae assimilate the other necessary nutrients needed for plants and animals. When algae die the y sink to the bottom where they are decomposed and the nutrients contained in or ganic matter are converted into inorganic form by bacteria. The decomposition pr ocess uses oxygen and deprives the deeper waters of oxygen which can kill fish a nd other organisms. Also the necessary nutrients are all at the bottom of the aq uatic ecosystem and if they are not brought up closer to the surface, where ther e is more available light allowing for photosynthesis for aquatic plants, a seri ous strain is placed on algae populations. Enhanced growth of aquatic vegetation or phytoplankton and algal blooms disrupts normal functioning of the ecosystem, causing a variety of problems such as a lack of oxygen needed for fish and shel lfish to survive. The water becomes cloudy, typically coloured a shade of green, yellow, brown, or red. Eutrophication also decreases the value of rivers, lakes , and estuaries for recreation, fishing, hunting, and aesthetic enjoyment. Healt h problems can occur where eutrophic conditions interfere with drinking water tr eatment.[2] Ocean waters Eutrophication is a common phenomenon in coastal waters. In contrast to freshwat er systems, nitrogen is more commonly the key limiting nutrient of marine waters ; thus, nitrogen levels have greater importance to understanding eutrophication problems in salt water. Estuaries tend to be naturally eutrophic because land-de rived nutrients are concentrated where run-off enters a confined channel. Upwell ing in coastal systems also promotes increased productivity by conveying deep, n utrient-rich waters to the surface, where the nutrients can be assimilated by al gae. The World Resources Institute has identified 375 hypoxic coastal zones in the wo rld, concentrated in coastal areas in Western Europe, the Eastern and Southern c oasts of the US, and East Asia, particularly Japan.[9] In addition to runoff from land, atmospheric fixed nitrogen can enter the open o cean. A study in 2008 found that this could account for around one third of the ocean s external (non-recycled) nitrogen supply, and up to 3% of the annual new ma rine biological production.[10] It has been suggested that accumulating reactive nitrogen in the environment may prove as serious as putting carbon dioxide in t he atmosphere.[11]
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Double Vertical Viso (K08A00B) : Cage Code: 65059 - Drawing No: SK08A00B - Revision: B - Sheet: 1 of 1Document1 pageDouble Vertical Viso (K08A00B) : Cage Code: 65059 - Drawing No: SK08A00B - Revision: B - Sheet: 1 of 1roy thomasNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Design of 115000 DWT TankerDocument160 pagesDesign of 115000 DWT Tankerroy thomasNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Sfi Detail CodeDocument175 pagesSfi Detail CodeAhmad Mustoin93% (15)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Mazagon Dock LTDDocument12 pagesMazagon Dock LTDroy thomasNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- MDL ReportDocument22 pagesMDL Reportroy thomas100% (1)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Cochin Shipyard Ltd.Document16 pagesCochin Shipyard Ltd.roy thomas100% (4)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Sky SailsDocument22 pagesSky Sailsroy thomas100% (2)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Naval Architect L2 PD MIDDocument3 pagesNaval Architect L2 PD MIDroy thomasNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
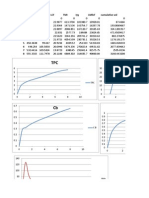
- T Awp Perimeter LCF Tmi Iyy Lmilcf Cumulative Vol 0 0.5 1 242.3197 1.5 280.4816 2 3 352.7904 4 5 450.3638 6 7 532.4416 109.5824 8 555.3315 111.7978Document15 pagesT Awp Perimeter LCF Tmi Iyy Lmilcf Cumulative Vol 0 0.5 1 242.3197 1.5 280.4816 2 3 352.7904 4 5 450.3638 6 7 532.4416 109.5824 8 555.3315 111.7978roy thomasNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Industrial TrainingDocument11 pagesIndustrial Trainingroy thomasNo ratings yet
- DWT Double Hull Bulk Carrier - Brief - SpecDocument10 pagesDWT Double Hull Bulk Carrier - Brief - Specroy thomasNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Let Us C - Yashwant KanetkarDocument743 pagesLet Us C - Yashwant Kanetkarsalahuddin.ayyubiNo ratings yet
- BMS of Dubai International AirportDocument4 pagesBMS of Dubai International AirportJomari Carl Rafal MansuetoNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Unemployment in IndiaDocument9 pagesUnemployment in IndiaKhushiNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- NREL Novel Electrolyzer Applications Providing More Than Just Hydrogen PDFDocument35 pagesNREL Novel Electrolyzer Applications Providing More Than Just Hydrogen PDFJosePPMolinaNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Vegetable Rates - 02-01-2021Document454 pagesVegetable Rates - 02-01-2021Saurabh RajputNo ratings yet
- ReagentsDocument12 pagesReagentsKimscey Yvan DZ SulitNo ratings yet
- Accessories 162-USDocument20 pagesAccessories 162-USعايد التعزيNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Mid Lesson 1 Ethics & Moral PhiloDocument13 pagesMid Lesson 1 Ethics & Moral PhiloKate EvangelistaNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Greek ArchitectureDocument16 pagesGreek ArchitectureXlyth RodriguezNo ratings yet
- Etl 213-1208.10 enDocument1 pageEtl 213-1208.10 enhossamNo ratings yet
- The Western and Eastern Concepts of SelfDocument3 pagesThe Western and Eastern Concepts of SelfTakumi Shawn Hinata100% (3)
- Nurtured Womb e BookDocument22 pagesNurtured Womb e BookSteph's Desserts100% (1)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- مستر رمضان عوضDocument24 pagesمستر رمضان عوضSamuel SaadNo ratings yet
- Chapter 1 - Part 1 Introduction To Organic ChemistryDocument43 pagesChapter 1 - Part 1 Introduction To Organic ChemistryqilahmazlanNo ratings yet
- DLP Physical Science Week1Document2 pagesDLP Physical Science Week1gizellen galvezNo ratings yet
- Third Quarter Pre-Test Mathematics 7 Directions: RDocument4 pagesThird Quarter Pre-Test Mathematics 7 Directions: RAhron RivasNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Buckthorpe Etal 23 Optimising Early Stage ACL Rehab ProcessDocument24 pagesBuckthorpe Etal 23 Optimising Early Stage ACL Rehab ProcessCole VincentNo ratings yet
- Boyle's Law 2023Document6 pagesBoyle's Law 2023Justin HuynhNo ratings yet
- DoomsdayDocument29 pagesDoomsdayAsmita RoyNo ratings yet
- 3rd Stage ComplicationsDocument84 pages3rd Stage ComplicationsDream100% (1)
- QIAGEN Price List 2017Document62 pagesQIAGEN Price List 2017Dayakar Padmavathi Boddupally80% (5)
- Burst Abdomen 3Document12 pagesBurst Abdomen 3Satvik BansalNo ratings yet
- Hydraulics Course FileDocument81 pagesHydraulics Course FileSwarna LathaNo ratings yet
- Topik 3 - Hazard Di Air Selangor, Penilaian Risiko Langkah Kawalan Rev1 2020 090320Document59 pagesTopik 3 - Hazard Di Air Selangor, Penilaian Risiko Langkah Kawalan Rev1 2020 090320Nuratiqah SmailNo ratings yet
- E Numbers Are Number Codes ForDocument3 pagesE Numbers Are Number Codes ForaradhyaNo ratings yet
- Boundary ScanDocument61 pagesBoundary ScanGéza HorváthNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Fe jkj101Document5 pagesFe jkj101ApezAnuarNo ratings yet
- Assignment 1 - Statistics ProbabilityDocument3 pagesAssignment 1 - Statistics ProbabilityAzel Fume100% (1)
- Charles Haanel - The Master Key System Cd2 Id1919810777 Size878Document214 pagesCharles Haanel - The Master Key System Cd2 Id1919810777 Size878Hmt Nmsl100% (2)
- Section 08630 Metal-Framed SkylightDocument4 pagesSection 08630 Metal-Framed SkylightMØhãmmed ØwięsNo ratings yet
- Bahasa Inggris PATDocument10 pagesBahasa Inggris PATNilla SumbuasihNo ratings yet