Professional Documents
Culture Documents
NMR Useful Tables
Uploaded by
Kelcie CambdOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
NMR Useful Tables
Uploaded by
Kelcie CambdCopyright:
Available Formats
Chapter 5 NMR Spectroscopy
Table 5.3 Typical ihl Chemical Shift Ranges in Organic Compounds
Group"
Tetramethylsilane (CH3)4Si
Methyl groups attached to Sp3 hybridised carbon atoms
Methylene groups attached to Sp3 hybridised carbon atoms
Methine groups attached to Sp3 hybridised carbon atoms
Acetylenic protons
Olefinic protons
Aromatic and heterocyclic protons
Aldehydic protons
6
lH
(ppm from TMS)
o
0.8 - 1.2
1.0 - 1.5
1.2 - 1.8
2-3.5
5-8
6-9
9 - 10
-OR protons in alcohols, phenols or carboxylic acids; -SR protons in thio1s; -NH
protons in amines or amides do not have reliable chemical shift ranges (see page 49).
Table 5.4 'a Chemical Shifts (6) for Protons in Common Alkyl Derivatives
44
CH3-X CH3CH2-X (CH3)2CH-X
X
- CH3 -CH3 - CH2-
---:- C
H3 'CH-
-:
-H
0.23 0.86 0.86 0.91 1.33
-CH=CH2
1.71 1.00 2.00 1.00 1.73
-Ph
2.35 1.21 2.63 1.25 2.89
-CI
3.06 1.33 3.47 1.55 4.14
-Br
2.69 1.66 3.37 1.73 4.21
-I
2.16 1.88 3.16 1.89 4.24
-OH
3.39 1.18 3.59 1.16 3.94
-OCH3
3.24 1.15 3.37 1.08 3.55
-O-Ph
3.73 1.38 3.98 1.31 4.51
-OCO-CH3
3.67 1.21 4.05 1.22 4.94
-OCO-Ph
3.89 1.38 4.37 1.36 5.30
-CO- CH3
2.09 1.05 2.47 1.08 2.54
-CO-Ph
2.55 1.18 2.92 1.22 3.58
-CO-OCH3
2.01 1.12 2.28 1.15 2.48
-NH2
2.47 1.10 2.74 1.03 3.07
-NH-COCH3
2.71 1.12 3.21 1.13 4.01
-C=N
1.98 1.31 2.35 1.35 2.67
- N02
4.29 1.58 4.37 1.53 4.44
Table 5.5
Chapter 5 NMR Spectroscopy
Approximate IH Chemical Shifts (0) for Olefinic Protons
C=C-H
OC=C-H =5.25 + CJgem + CJcis + CJtrans
Xtrans", / X
gem
C=C
/ "
Xcis H
X
CJgem CJcis CJtrans
,
.'
-H
0.0 0.0 0.0
-alkyl
0.45 -0.22 -0.28
-aryl
1.38 0.36 -0.07
- C H = C ~
1.00 -0.09 -0.23
-CH=CH-conjugated
1.24 0.02 -0.05
-C=C-H
0.47 0.38 0.12
-CO-R
1.10 1.12 0.87
-CO-OH
0.80 0.98 0.32
-CO-OR
0.78 1.01 0.46
-C=:N
0.27 0.75 0.55
-CI
1.08 0.18 0.13
-Br
1.07 0.45 0.55
-OR
1.22 -1.07 -1.21
-NRz
0.80 -1.26 -1.21
Table 5.6 gives characteristic IH chemical shifts for the aromatic protons in benzene
derivatives. To a first approximation, the shifts induced by substituents are additive.
So, for example, an aromatic proton which has a -N0
2
group in the para position and
a -Br group in the ortho position will appear at approximately 7.82 ppm
[(7.26 + 0.38(p-N0
2
) + 0.18(o-Br)].
Tables 5.7 gives characteristic chemical shifts for IH nuclei in some polynuclear
aromatic compounds and heteroaromatic compounds.
45
Chapter 5 NMR Spectroscopy
Table 5.6
IH Chemical Shifts (0) for Aromatic Protons in Benzene
Derivatives Ph-X in ppm Relative to Benzene at 0 7.26 ppm
(positive sign denotes a downfield shift)
X ortho meta para
-H
0.0 0.0 0.0
-CH3
-0.20 -0.12 -0.22
-C(CH
3
h
-0.03 -0.08 0.20
-CH=CH2
0.06 -0.03 -0.10
-C=C-H
0.16 -0.04 -0.02
-CO-OR
0.71 0.11. 0.21
-CO-R
0.62 0.14 0.21
-OCO-R
-0.25 0.03 -0.13
- OCH3
-0.48 -0.09 -0.44
-OH
-0.56 -0.12 -0.45
-CI
0.03 -0.02 -0.09
-Br
0.18 -0.08 -0.04
-C:::N
0.36 0.18 0.28
- N0 2
0.95 0.26 0.38
-NR2
-0.66 -0.18 -0.67
-NH2
-0.75 -0.25 -0.65
Table 5.7 IH Chemical Shifts (0) in some Polynuclear Aromatic Compounds
and Heteroaromatic Compounds
7.71
7.81
7.46
8.31 7.91
CCO
"-':::: "-':::: "-':::: 7.39
~ ~ ~
Jh.8.12
l r U - ~ II _ ~ 7.82
8.93 7.88
46
6.30
o 7.40
a
7.04
o 7.19
S
7.46
o
N
7.06
8.50
Table 5.8
Chapter 5 NMR Spectroscopy
Typical ifl - IH Coupling Constants
Group
CH
3CHzCHzCH3
CH
3CHzCHzCH3
CH
3CHzCHzCH3
HzC=C=C=CH
z
HzC=CH-CH=CH
z
J(Hz)
ZJ
HH:::::
-16
3J
HH
= 7.2
4J
HH
= 0.3
5J
HH
= 7
5J
HH
= 1.3
4J
HH
= 1.5
Signal Multiplicity - the n+l rule. Spin-spin coupling gives rise to multiplet
splittings in IH NMR spectra. The NMR signal of a nucleus coupled to n equivalent
hydrogens will be split into a multiplet with (n+l) lines. For simple multiplets, the
spacing between the lines (in Hz) is the coupling constant. The relative intensity of
the lines in multiplet will be given by the binomial coefficients of order In'
(Table 5.9).
Table 5.9 Relative Line Intensities for Simple Multiplets
multiplicity relative line multiplet
n n+l intensities name
0 1 1 singlet
1 2 1 : 1 doublet
2 3 1 : 2 : 1 triplet
3 4 1 : 3 : 3 : 1 quartet
4 5 1:4:6:4:1 quintet
5 6 1 : 5 : 10: 10: 5 : 1 sextet
6 7 1 : 6 : 15 : 20 : 15 : 6 : 1 septet
7 8 1 : 7 : 21 : 35 : 35 : 21 : 7 : 1 octet
8 9 1 : 8 : 28 : 56 : 70 : 56 : 28 : 8 : 1 nonet
51
APPENDIX B 191
EFF'ECT ON CHEMICAL SHIFTS BY TO OR THREE DIRECTLY
APPENDIX B ATACHED FUNCTIONAL GROUPS
Y-CH -Z and Y-CH-Z
2
W
The chemical shift of a methylene group attached to two
functional groups can be calculated by means of the
substituent constants (0 values) in Tablc B. 1. Shoolery's rule*
states that the sum of the constants for the attached functional
groups is added to [ 0.23, the chemical shift for CH4:
The chemical shift for the methylene protons, of
C6H,CHzBr, for example, is calculated from the 0 values in
Table 8.1.
0.23
(Ph
= 1.85
(Sr = 2.33
( 4.41 Found, ( 4.43
Shoolery's original constants have been revised and
extended in Table 8.1. Te observed and calculated chemical
shifts for 62% of the samples tested were within 0.2 ppm,
92% within 0.3 ppm. 96% within 0.4 ppm, and 99%
within 0.5 ppm.t Table B. l contains substituent constants
(Friedrich and Runkle, 1984) for the more common functional
* Shoolery, J.N. (1959). Varian Technicallnforratiol Bulletin, Vol 2,
No.3. Palo Alto, CA: Varian Associates.
t Data from Friedrich, E.c., and Runkle, K.G. (1984). 1. Cher. Educ.
61,830;(1986)63,127.
TABLE B.1 Substituent Constants for Alkyl Methylene
(and Methyl) Protons.
Substituent Substituent
Constants Constants
YorZ
(0
YorZ
(0)
-H 0.34 -OC(=O)R 3.01
-CH
3
0.68 -OC(=O)Ph 3.27
-C-C 1.32 -C(=O)R 1.50
-C=C 1.44 -C(=O)Ph 1.90
Ph 1.83 -C(=O)OR 1.46
-CFz 1.12 -C(=O)NRz(Hz) 1.47
-CF3 1.14 -C=N 1.59
-F 3.30 -NRz(Hz) 1.57
-Cl 2.53 -NHPh 2.04
-Br 2.33 -NHC(=O)R 2.27
-I 2.19 -N] 1.97
-OH 2.56 -NOz 3.36
-OR 2.36 -SR(H) 1.64
-OPh 2.94 -OS02R 3.13
groups. Note that chemical shifts of methyl protons can be
calculated by using the constant for H (0.34). For example
H-CH z-Br is equivalent to CH3Br.
Tables B.2a, B.2b, and B.2c: Chemical
Shift Correlations for Methine Protons
Table B. 2a gives the substituent constants* to be used with
the formulation
8 CHXYZ 2.50 + (x + (y
+
(z
which is satisfactory if at leas t two of the substituents are
electron-withdrawing groups. In other words, only a single
substituent may be an alkyl group (R). Within these limits,
the standard error of estimate is 0.20 ppm. For example, the
chemical shift of the methine proton in
OEt
CH -CH-OEt
3
is calculated from Table 8.2a as follows:
8 2.50 + 1.14 + 1.14 + 0.00 4.78
Te found value is 4.72.
Tables B.2b and B.2c are used jointly for methine pro
tons that are substituted by at least two alkyl groups
"Bell, H.M., Bowles, D.B. and Senese, F (1981). Org. Magn. Resol.,
16,285. With permission.
TABLE B.28 Substituent Constants for Methine Protons.
Group
(0)
-F 1.59
-Cl 1.56
-Br 1 .53
-N02 1.84
-NH2 0.64
-NH +
3
1.34
-NHCOR 1.80
-OH,-OR 1.14
-OAr 1.79
-OCOR 2.07
-Ar 0.99
-C=C 0.46
-C
-
C 0.79
-C=N 0.66
-COR,-COOR,-COOH 0.47
-CONHz 0.60
-COAr 1.22
-SH, -SR 0.61
-S02R 0.94
-R 0
192 CHAPTER 3 PROTON NMR SPECTROMETRV
TABLE B.2b Observed Methine Proton Chemical Shifts
of Isopropyl Derivatives.
(CH3hCHZ (CH3hCHZ
o (ppm) o (ppm)
Z obs Z obs
H 1.33 HO 3.94
H3C 1.56 RO 3.55
R 1.50 C6HsO 4.51
XCH2 1.85 R(H)C(=O)O 4.94
R(
H
)C(=O) 2.54 C6HSC(=O)O 5.22
C6HSC(=O) 3.58 F3CC(=O)O 5.20
R(H)OC(=O) 2.52 ArS020 4.70
R2(H2)NC(=O) 2.44
C
o
H
s
2.89 R(H)S 3.16
R2(H2)C=CR(H) 2.62 RSS 2.63
R(H)C C 2.59
N=C 2.67 F 4.50
CI 4.14
R2(H2)N 3.07 Br 4.21
R(H)C(=O)NH 4.01 1 4.24
02N 4.67
(or other groups of low polarity). Friedrich and Runkle pro
posed the relationship
0CHXYZ
O(CH,)zCHZ
xy
in which the X and Y substituents are alkyl groups or other
groups of low polarity. Te Z susbstituent covers a range of
polarities . .xy is a correction factor. The relationship states
that the chemical shift of a methine proton with at least two
low-polarity groups is equivalent to the chemical shift of an
isopropyl methine proton plus correction factor.
The substituent constants for a Z substituent on an
isopropyl methine proton are given in Table B.2b. Te y
correction factors are given in Table B.2e.
The following example illustrates the joint use of Tables
B.2b and B.2e, with CH3, CH=CH2, and CoHs as sub
stituents. The most polar substituent is always designated Z.
TABLE B.2e Correction Factors for Methine
Substituents of Low Polarity.
Cyclic
Open-Chain Methine
Methine Proton Proton
Systems
a
xy Systems
a
xy
z
I
CH)-CJ-CH3 0.00 -1.0
Z
Z
I
I
l
CH)-CJ: -R -0.20 +0.40
Z
I
R-CH-R -0.40 +0.20
Z
,
I
CH}-C! -CH2X +0.20 monosub. -0.20
axial H -0.45
Z
I
CH3-C!:-CH=CH2 +0.40 equat. H +0.25
Z
I
CH3-CH-C6Hs + 1.15 0.00
Z
I
R-CH-Cr
H
s +0.90 0.00
C6H
S
I
From Table B.2b, (
2.89 for CH3-CH-CH].
From Table B.2e,.y = 0.00 for CH} . .y 0.40 for
CH=CHz
C6H-
I )
Terefore, 0 CH}-Ct-CH=CH2
2.89 + 0.00 +
0.40 3.29 (Found: ( 3.44).
TABLE C.1
TABLE C.2
Chemicals Shifts in Alicyclic Rings.
`
0
0.22
1.96
1.51
1.44
1.54
`
1.65
1.96 3.03
o
2 06
.
38
1.8
2.02
1.8
Chemical Shifs in Heterocyclic Rings.
2.54
1.62
\
N
H 0
.
03
2.27
\
S
2.72 4.73
H 2.38
2.23 3.54
3.17 3.43
1.85
3.75
o
1.59
2.75
N
H 2.01
_1.93
2.82
S
R O
3.9-4.1
H o
5.90
o
4.75-4.90
3 01
2.08 38
2.31
o
o
1
.51
3.52
1.50
1.50
2.74
N
H 1.84
.3
S
3.00
O
2
4.70
1.68
3.80
1.62
1.62
4.06
2.27
1.78
3
0
1.94
1.52 1.52
f
e
B
,
1.90
3.70
S
O
2
)
S
5
o
APPENDIX c 193
194 CHAPTER 3 PROTON NMR SPECTROMETRY
(See Table D.1)
5.25 + Zgem + Zcs + Ztrans
For example, the chemi cal shifts of the alkene
protons in
are calculated:
Ha
C
6HSgem
o R
trans
H
b
OR/em
C6HStran.,
TABLE D.1 Substituent Constants (
Z
) for Chemical Shifts of Substituted Ethylenes.
Z Z
Substituent R gem cis trans Substituent R gem cis
-H 0 0 0
H
-Alkyl 0. 44 -0.26 -0.29 / 1.03 0.97
-Alkyl-ringa 0.71 -0.33 -0. 30
-C=O
-CH20, -CH21 0.67 -0.02 -0.07
N
-CH2S 0.53 -0o15 -0.15
/
1.37 0.93
-C=O
-CH2CI, -CH2Br 0.72 0.12 0.07
-CH2N 0.66 -0.05 -0.23
/
CI
1.10 1.41
-C=C 0.50 0.35 0.10 -C=O
-C
-
N 0.23 0.78 0.58 -OR, R: aliph 1.18 -1.06
-C=C 0.98 -0.04 -0.21 -OR, R: conj
b
1.14 -0.65
-C=Ccon? 1.26 0.08 -0.01 -OCOR 2.09 -0.40
-C=O 1.10 1.13 0.81 -Aromatic 1.35 0.37
-C=Oconl 1.06 1.01 0.95 -Cl 1.00 0.19
-COOH 1.00 1.35 0.74 -Br 1.04 0.40
R
-COOHconjb 0.69 0.97 0.39
/
0.69 -1.19
-N R:aliph
"
R
R
-COOR 0.84 1.15 0.56
/
2.30 -0.73
-N R:conjb
"
R
-COORconjb 0.68 1.02 0.33 -SR 1.00 -0.24
-S02 1. 58 1.15
Ie
Alkyl ring indicates that the double bond is part of the ring R II.
"- e
1.35
~
1 o 28
0.07
1.18
-0.10
1.08
trans
1.21
0.35
0.99
-1.28
-1o05
-0.67
-0.10
0.03
0.55
-1.31
-0.81
-0.04
0. 95
b The Z factor for the conjugated substituent is used when either the substituent or the double bond is further conjugated
with other groups.
Source: Pascual C, Meier, 1, and Simon, W. (1966) Helv. Chim. Acta, 49, 164.
5.25
0.07
85.32
5.25
1.08
86.33
TABLE 0.2 Cemical Shifts of Miscellaneous Alkenes
R = C(=O)OCH3
----------------
2.12 "
.
. .
. . . . . .
1.84 5.62
' 1
.
. .
0
6
.
42 1.65
2.02
7
9
7.36
6
.
7
.82
.
0
4
.
20
0
3
.97 0 6.37
7.53 7.72
0
.
7.32
R C(=O)CH3
2.06
.
. .
1.86
5
.
9
7
R = OC(=O)CH3
H3C .
1.65 6.67
2.57
0.92
5.95 2.28
2.20 7.71
_
6.40
.
3
2.13
4.63
3
34 ,."
0
5.1 J
6.15 7.63
piperitone linalool a-terpinene
TABLE 0.3 Chemical Shifts of
A
lkyne Protons
HC=CR
HC=C-C=CR
HC-C-Ph
1.73-1.88
1.95
2.71-3.37
HC=C-COH
HC=CH
HC=C-CH=CR2
TABLE 0.4 Chemical Shifts of Protons on Fused
A
romatic Rings
7.81
7.46
,
8.69
7
.
81
2.23
1.80
2.60-3.10
APPENDIX D 195
196 CHAPTER 3 PROTON NMR SPECTROMETRY
CHE' MICAL SHIFTS OF PROTONS ON MONOSUBSTITUTED
BENZENE RINGS
Benene a
CH, (omp)
CH,CH2 (omp)
(CH3hCH (omp)
(CH,),C o,m,p
C=CH2 (omp)
CaH 0, (mp)
Penyl 0, m, p
CF, (omp)
CH2Cl (omp)
CHCl2 (omp)
CC1, 0, (mp)
:
CH20H (omp)
CH20R (omp)
CH2O(=O)H, (omp)
CH2NH2 (omp)
F m,p.o
Cl (omp)
Br 0, (pm)
Io,p,m :
OH m,p,o
OR m,(op)
OC( -O)CHa m,p,o
OTsb (mp), 0
CH(-O)o,p,
.
C(=O)H, 0, (mp)
C(=O)H 0, p, m
C(=O)R 0, p, m
:
C(-O)l 0, p, m
.
.
DN (omp)
N2 m,p,o
N(CH,h m(op)
NHC(=O)R o,m,p
.
.
Nj 0 (mp)
N020,p,
.
. .
SR(omp)
N-C-O (op)
:
:
.
.
:
:
:
;
G
:
.
:
:
:
B
B
:
:
M B
B
:
:
M
M
M
& B
M
:
M
:
:
:
:
: :
.
. .
.
.
The benzene ring proton is at 07.27, from which the shift increments are calculated as shown at the end of Section 3.4.
b OTS p-toluenesulfonyloxy group.
APPENDIX E 197
TABLE 0.5 Chemical Shifts of Protons on Heteroaromatic Rings
-
0
3.88
..
H
.,
0
.
7.30
S
7.10
-0
..
,
.
3
.
88
6.1 .0
S
H
0 0
......
...
.
7
.3 0.57 .
.
H \
H -H.O
-
-11.0
\\ NH
7.54
.
,
.
_
_
.
6
.7
/
_ N
. ,. 27
.
.
.
.
7.
3
7
6.7
6
-10.0
6.LO .
7.12 7.18
TABLE 0.6 Chemical Shifts of HC=O, HC=N, and HC(O)3 Protons.
RCH=O
PhCH=O
RCH=CHCH=O
9.70
9.98
9.78
HC(=O)OR
HC(=O)NR2
8.05
8.05
5.00
RCH=NOHcis
RCH=NOH trans
N0
2
R
y
'
H N0
2
7.25
6.65
6.05
8O
N EFFECTS
N E
S 17 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 0
Proton Class
OH Carboxylic acids
Sulfonic acids
Phenols
Phenols (intramolecular H bond)
in DMSO
Alcohols
Enols (cyclic a-diketones) ..
Enols (f-diketones)
Enols (f-ketoesters) l
inDMSO, . in acetone
Water I
H H
.
Oximes
NH2 and NHR Alkyl and cyclic amines
Aryl amines
Amides
Urethanes
Amines in trifluoroacetic acid
SH Aliphatic mercaptans H
Thiophenols t-
S 17 16 l5 14 13 12
11
10 9 8 7 6 5 4 3 2
1
0
Solvent CDCI). Chemical shifts within a range are a function of concentration.
b See Section 3.6.1.2.
198 CHAPTER 3 PROTON NMR SPECTROMETRY
APPFNDIX F PROT0N 8PIN.
Type
H
,
/
a
/
C
"
H"
CHa -CH" (free rotation)
I
CH -C-CH
a
I
h
ax-ax
ax-eq
eq-eq
Q
H
,
H
b
( cis or trans)
H
a
H"
Jab (Hz)
0-30
6-8
0-1
6-14
0-5
0-5
cis5-10
trans 5-10
Jab
Typical
12-15
7
0
8-10
2-3
2-3
Type
H
a
H"
'=C
/
/
"
CH
a
CH
h
`=;
/ "
CH
a
`C
/
/ "
H"
CHb
"
C
=
C
/
/ "
H
a
H
a
CH,)
'-
/
/
-C"
C=CH-CH C
L b
H H
""
/
"
C=C
CH,,-C-CH
b
c
H
,
cis 4-12
-CHa-C-C-CHb-
trans 2-10
H
/J
(cis or trans)
H
b
(cis or trans)
CH
a
-OHb (no exchange)
/
CH,,-
CHb
C=CHa-CHb
H
a
`;
/ "
H
C=C
H
b
H
"
cis 7-13
trans 4-9
4-10
1-3
5-8
12-18
0-3
5
2-3
6
17
0-2
H
a
Hi
HlI
H
b
0
H
I)
H
a
0
H
a
6 ,
N
0
] (ortho)
] (meta)
]
(para)
] (2-3)
] (3-4)
] (2-4)
] (3-5)
] (2-5
)
] (2-6)
] (2-3)
] (3-4)
] (2-4)
] (2-5)
Jab
(Hz)
Jab
Typical
6-12 10
0-3 1-
2
4-10 7
0-3 1.5
0-3 2
9-13 10
3 member 0.5-2.0
4 member 2.5-4.0
5 member 5.1-7.0
6 member 8.8-11.0
7 member 9-13
8 member 10-13
2-3
2-3
6
4
2.5
6-10
1-3
3
0-1 -0
5-6 5
7-9 8
1 -2 1. 5
1-2 1.5
0-1 1
0-1 -0
1.3-2.0 1 .8
3.1-3.8 3.6
0-1 -0
1-2 1.5
T
yp
e
[
s
H
4
s
N
6 .
N
J (2-3)
J(3-4)
J(2-4)
J (2-5)
J (1-3)
J (2-3)
J (3-4)
J(2-4)
J (2-5)
J (4-5)
J (2-S)
J (2-4)
J (4-6)
J (4-5)
J (2-4)
J (2-5)
Jab (Hz)
Jab
T
yp
ical
4.9-6.2 5.4
3.4-5.0 4.0
1.2-1.7 1.5
3.2-3.7 3.4
2-3
2-3
3-4
1-2
1.5-2.5
46
1-2
0-1
2-3
3-4
-0
1-2
T
yp
e
Proton -Carbon-13
(See Tables 5.17,5.18)
Proton -Fuorine
H
"" /
"
/
C
""
F
{)
CHu-CF
b
/
/
CH,,-C-CFh
I I
=C
/
/ "
H
a
F
h
H
a"
/
C=C
/ "
F
F
b
_
QRC-C-CHoFy
.,
{ -
Proton -Phosphorus
o
/PH
(CHJ)
J
P
(CH
3)JP=0
(CHJCH2)JP
(CH3CH2)JP=0
o
CH3P (OR)2
o
CRCP(OR)o
CH30P (OR)2
P[N(CHJ)2b
O=P[N(CH3hb
630-707
2.7
13.4
0.5 (HCCP) 13.7 (HCP)
11.9 (HCCP) 16.3 (HCP)
10-13
15-20
10.5-]2
8.8
9.5
Source: Complied by Varian Associates. Absolute values. Reproduced with permission.
APPENDIX F 199
Jab (Hz)
44-81
3-25
0-4
1-8
12-40
() 6-10
mS-6
p2
a
-
4.3
1-
48
Jab
T
yp
ical
200 CHAPTER 3 PROTON NMR SPECTROMETRY
CHEMICAL SHIFTS AND MULTIPLICITIES OF RESIDUAL
PROTON' S IN COMMERCIALL AI'LABLE DEUTERATED
APPENDIX G SOLVENTS (MERCK & CO., INC.)
Compounda Molecular Weight
Acetic acid-d4
64.078
Acetone-do
64.117
Acetonitrile-d]
44.071
Benzene-do
84.152
Chloroform-d
120.384
Cyclohexane-d
12
96.236
Deuterium oxide
20.028
1,2-Dichloroethane-d4
102.985
Diethyl-dl ether
84.185
Diglyme-d14
148.263
N, N-Direthylforraride-d7
80.138
Dimethyl-do sulphoxide
84.170
p-Dioxane-dH
96.156
Ethyl alcohol-do (anh)
52.106
Glyme-dl
100.184
Hexafuroacetone deuterate
198.067
HMPT-d
18
197.314
Methyl alcohol-d4
36.067
Methylene chloride-d2
86.945
Nitrobenzene-d,
128.143
0. (multiplet)
11.53 (1)
2.03 (5)
2.04 (5)
1.93 (5)
7.15 (br)
7.26(1)
1.38 (br)
4.63 (ref DSSY
4.67 (ref. TSP),
3.72 (br)
3.34 (m)
1.07 (m)
3.49 (br)
3.40 (br)
3.22 (5)
8.01 (br)
2.91 (5)
2.74 (5)
2.49 (5)
3.53 (m)
5.19 (1)
3.55 (br)
1.11 (m)
3.40(m)
3.22 (5)
5.26 (1)
2.53 (2 x 5)
4.78(1)
3.30 (5)
5.32 (3)
8.11 (br)
7.67 (br)
7.50 (br)
" Purity (Atom % D) up to 99.96 % (100 % ) for several solvents.
Compounda Molecular Weight
Ni tromethane-d]
64.059
Isopropyl alcohol-dB
68.146
Pyridine-ds
84.133
Tetrahydrofuran-dg
80.157
Toluene-ds
100.191
Trifuoroacetic acid-d
115.030
2,2,2-Trifuoroethyl alcohol-d]
103.059
(multiplet)
4.33 (5)
5.12 (1)
3.89 (br)
1.10 (br)
8.71 (br)
7.55 (br)
7.19 (br)
3.58 (br)
1.73 (br)
7.09 (m)
7.00 (br)
6.98 (m)
2.09 (5)
11.50 (1)
5.02 (1)
3.88 (4 X 3)
{'Te residual proton consists of one proton of each kind in an otherwise completely deuterated molecule. For example, deuterated acetic acid has two
different kinds of residual protons: CD2H-COOD and CD]-COOH. Te CD2H proton, coupled to two D nuclei is at D 2.03 with a multiplicity of 5
(i.e .. 2nl + 1 2 X 2 X 1 + 1 5). Te carboxylic proton is a singlet at D 11.53.
DSS is 3-trimethylsilyl)-I-propane sulfonic acid, sodi um salt. TSP is sodium-3-tr imethylpropionate-2,2,3,3-d4. Both are reference standards used
in aqueous solutions.
You might also like
- PMR Spectroscopy: Solved Problems Volume : IIFrom EverandPMR Spectroscopy: Solved Problems Volume : IIRating: 5 out of 5 stars5/5 (3)
- Absorption Spectra and Chemical Bonding in ComplexesFrom EverandAbsorption Spectra and Chemical Bonding in ComplexesRating: 2.5 out of 5 stars2.5/5 (2)
- Spectroscopic Solutions of StructureDocument21 pagesSpectroscopic Solutions of StructureKassimNo ratings yet
- Guide To Solving Spectroscopy ProblemsDocument4 pagesGuide To Solving Spectroscopy ProblemsJen100% (1)
- Experiment 2. Separation of Compounds by Paper ChromatographyDocument11 pagesExperiment 2. Separation of Compounds by Paper ChromatographybidinNo ratings yet
- NMR Solving StrategyDocument2 pagesNMR Solving Strategysorrow Lemon100% (1)
- AC 101 Unit 1 Titrimetric AnalysisDocument90 pagesAC 101 Unit 1 Titrimetric AnalysisRishabh Kumar Singh100% (1)
- Aromaticity CompleteDocument104 pagesAromaticity Completewahidalwahdi100% (1)
- 1 IR NMR Practice ProblemsetDocument12 pages1 IR NMR Practice ProblemsetJustin BuiNo ratings yet
- Molecular RearrangementsDocument9 pagesMolecular RearrangementsDhanaswamy Ilangeswaran67% (3)
- Module8 PDFDocument40 pagesModule8 PDFFaizan AhmadNo ratings yet
- Kuliah MG 9 Racemix Mixture and ResolutionDocument184 pagesKuliah MG 9 Racemix Mixture and ResolutionBowo Aank ApriantoNo ratings yet
- Silverstein Chapter 1 Mass SpectrometryDocument71 pagesSilverstein Chapter 1 Mass SpectrometryNikita GroverNo ratings yet
- AromaticityDocument12 pagesAromaticityV G Viju KumarNo ratings yet
- Molecular Spectroscopy: BackgroundDocument45 pagesMolecular Spectroscopy: Backgroundsavvy_as_98100% (1)
- NMR SpectrosDocument185 pagesNMR SpectrosBathir JafarNo ratings yet
- (UV Vis) SpectrosDocument4 pages(UV Vis) SpectrosGarion Charles0% (1)
- Huckel Theory For Conjugated Systems: CH 105: Organic ChemistryDocument72 pagesHuckel Theory For Conjugated Systems: CH 105: Organic ChemistryRaunaq Bhirangi100% (1)
- Retrosynthetic Analysis PDFDocument6 pagesRetrosynthetic Analysis PDFNoleNo ratings yet
- Clusters and Catenation in P-Block: Allotropes of CarbonDocument15 pagesClusters and Catenation in P-Block: Allotropes of Carbonrajender kumarNo ratings yet
- Lecture Notes 2 Nano MaterialsDocument21 pagesLecture Notes 2 Nano MaterialsHuzaifa ShabbirNo ratings yet
- Mass Spectra Worksheet 1Document5 pagesMass Spectra Worksheet 1scribdfreepdfNo ratings yet
- Elimination ReactionsDocument7 pagesElimination ReactionsIrfan IslamyNo ratings yet
- Practice Problems For Physical Chemistry 2Document1 pagePractice Problems For Physical Chemistry 2Fatima CellonaNo ratings yet
- Aromaticity NotesDocument10 pagesAromaticity NotesVirendra Singh Rajput100% (1)
- Spec Prob Set 315 CurrentDocument20 pagesSpec Prob Set 315 CurrentUmang Agarwal57% (7)
- 13C NMR SpectrosDocument16 pages13C NMR Spectrosapi-3723327100% (4)
- Metal Complexes or Coordination Compounds: Kfecn 4K Fe CNDocument90 pagesMetal Complexes or Coordination Compounds: Kfecn 4K Fe CNPavan Boro100% (1)
- The Transition Metals, The Lanthanides and The AntinidesDocument21 pagesThe Transition Metals, The Lanthanides and The AntinidesApril CruzNo ratings yet
- Chap21 Skoog PotentiometryDocument55 pagesChap21 Skoog PotentiometryMarielle Perejon100% (1)
- Organometallic Chemistry: CH 431 MFT CH 13Document37 pagesOrganometallic Chemistry: CH 431 MFT CH 13Vaittianathan MahavapillaiNo ratings yet
- MOT UploadDocument36 pagesMOT UploadSarthak Singh100% (1)
- 01 GC TheoryDocument71 pages01 GC Theory03ASEPJAELANINo ratings yet
- Report NMRDocument13 pagesReport NMRsarahNo ratings yet
- FMO LectureDocument14 pagesFMO Lecturebooks4free23No ratings yet
- Questions On StereochemistryDocument2 pagesQuestions On StereochemistryShilajit BaruaNo ratings yet
- UV Spectroscopy 2016Document87 pagesUV Spectroscopy 2016M Mudassar AslamNo ratings yet
- Organometal Chem PDFDocument55 pagesOrganometal Chem PDFSushmita Dey100% (1)
- IR - HNMR ProblemsDocument33 pagesIR - HNMR Problemsbsakaly112100% (1)
- BW Mass Spectrometry - ZeeshanDocument59 pagesBW Mass Spectrometry - ZeeshanAdnan RoonjhaNo ratings yet
- NMR Practice ProblemsDocument9 pagesNMR Practice ProblemsVivek AgrahariNo ratings yet
- SET-NET Pericyclic ReactionsDocument61 pagesSET-NET Pericyclic ReactionsBapu ThoratNo ratings yet
- Organic Structure Elucidation WorkbookDocument498 pagesOrganic Structure Elucidation WorkbookKajan Muneeswaran75% (4)
- Lucas Test PDFDocument3 pagesLucas Test PDFciciNo ratings yet
- NMR SpectrosDocument29 pagesNMR Spectroshareesh13h100% (1)
- CH 431 Lab ManualFullDocument28 pagesCH 431 Lab ManualFullHân BảoNo ratings yet
- Lab #1: Absorption Spectra of Conjugated Dyes: E E E EDocument5 pagesLab #1: Absorption Spectra of Conjugated Dyes: E E E EIreneVeladoNo ratings yet
- Isolobal AnalogyDocument4 pagesIsolobal Analogyindu priyaNo ratings yet
- Journal of Electroanalytical Chemistry 609 (2007) 17-26Document10 pagesJournal of Electroanalytical Chemistry 609 (2007) 17-26Alex B-RomeroNo ratings yet
- Gravimetric Analysis and Precipitation - TitrationsDocument34 pagesGravimetric Analysis and Precipitation - TitrationsElvinNo ratings yet
- Electrochemistry Exercise SolutionDocument22 pagesElectrochemistry Exercise SolutionGOURISH AGRAWALNo ratings yet
- NMR HandoutDocument23 pagesNMR HandoutVirendra Singh RajputNo ratings yet
- 3820 Lecture Chapter - 3 - Part1 - 2004 PDFDocument15 pages3820 Lecture Chapter - 3 - Part1 - 2004 PDFPhuongUblMyNo ratings yet
- CAIE Chemistry A-Level: 24: ElectrochemistryDocument8 pagesCAIE Chemistry A-Level: 24: ElectrochemistryahumanbeinginearthNo ratings yet
- Isolation Purification and Identification of CurcuminoidsDocument5 pagesIsolation Purification and Identification of CurcuminoidsNguyenVan HanNo ratings yet
- NMR 1Document49 pagesNMR 1Jyoti ChaturvediNo ratings yet
- Aldehydes and KetonesDocument5 pagesAldehydes and KetonesBaji Babu BejjankiNo ratings yet
- Chemistry II (Organic) Heteroaromatic Chemistry Lectures 2 & 3Document25 pagesChemistry II (Organic) Heteroaromatic Chemistry Lectures 2 & 3Subhabrata MabhaiNo ratings yet
- GRE Sub 化学题 (太傻整理)Document30 pagesGRE Sub 化学题 (太傻整理)Alisa100% (1)
- Experiment 1 Solubility of Organic CompoundsDocument3 pagesExperiment 1 Solubility of Organic CompoundsIshaa IluminNo ratings yet
- Organic Compounds: Are They Useful?: Science 9 Week 4 WorksheetsDocument4 pagesOrganic Compounds: Are They Useful?: Science 9 Week 4 WorksheetsGEROME REY LINONo ratings yet
- PYQ of Organic Nomenclature NEET 2022Document25 pagesPYQ of Organic Nomenclature NEET 2022Saloni tyagi100% (2)
- 26 Halogen Derivatives Formula Sheets Getmarks AppDocument10 pages26 Halogen Derivatives Formula Sheets Getmarks AppsinghrishxbhNo ratings yet
- 11 Alcohols Phenols and EthersDocument6 pages11 Alcohols Phenols and EthersVansh VaibhavNo ratings yet
- Heterocycles Essentials1-2009Document2 pagesHeterocycles Essentials1-2009Aravindan NatarajanNo ratings yet
- ReasoningDocument4 pagesReasoningAayush MishraNo ratings yet
- Organic Chemistry - Chapter 19 - NitrilesDocument5 pagesOrganic Chemistry - Chapter 19 - NitrilesSairille ManejaNo ratings yet
- ORGANIC Chemistry: 1. S.No. Compound Aromatic Anti-Aromatic Non-AromaticDocument6 pagesORGANIC Chemistry: 1. S.No. Compound Aromatic Anti-Aromatic Non-AromaticTarun SoniNo ratings yet
- Alcohols & Ethers Exercise - IDocument5 pagesAlcohols & Ethers Exercise - IVasudev ArchakNo ratings yet
- Recognizing Endo and Exo - Master Organic ChemistryDocument9 pagesRecognizing Endo and Exo - Master Organic ChemistryashishNo ratings yet
- Hydrocarbons QuestionsDocument5 pagesHydrocarbons QuestionsBhakti Nath MishraNo ratings yet
- Alkynes 1Document27 pagesAlkynes 1Irfan GumelarNo ratings yet
- Al KanesDocument35 pagesAl Kanessimonatics08No ratings yet
- Chemistry Xii NAME: - : Alcohol, Phenol & EtherDocument1 pageChemistry Xii NAME: - : Alcohol, Phenol & EtherSahir Hemnani100% (1)
- Chapter 24 - Organic ChemistryDocument13 pagesChapter 24 - Organic Chemistrymaniz442No ratings yet
- Organic Chemistry: Dr. Omar Mohammed YahyaDocument15 pagesOrganic Chemistry: Dr. Omar Mohammed YahyaCover SongsNo ratings yet
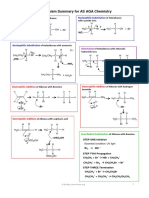
- Mechanism Summary For AS AQA Chemistry: HO: NCDocument4 pagesMechanism Summary For AS AQA Chemistry: HO: NCjohn mNo ratings yet
- T12 Introduction To Organic Chemistry 27-34Document8 pagesT12 Introduction To Organic Chemistry 27-34饶宝珍No ratings yet
- Lecture 2 - S1 - 96 PDFDocument15 pagesLecture 2 - S1 - 96 PDFAnonymous dSQiRGNo ratings yet
- Transition Metal CatalysisDocument3 pagesTransition Metal Catalysisapi-250366166No ratings yet
- P-101 AlkynesDocument10 pagesP-101 AlkynesNISARG PATKARNo ratings yet
- PART TEST-1 (Ogranic Chemistry) PDFDocument6 pagesPART TEST-1 (Ogranic Chemistry) PDFKinshuk RastogiNo ratings yet
- Haloalkanes and Haloarenes-15 Mar 23Document5 pagesHaloalkanes and Haloarenes-15 Mar 23akshat.sh2021No ratings yet
- 8-Birch Reduction PDFDocument7 pages8-Birch Reduction PDFVENUGOPALARAO100% (1)
- LipidsDocument8 pagesLipidsBILL LLONARD RESURRECCIONNo ratings yet
- Alkanes, Alkenes, Alkunas and Cycloalkanes: Discussion GroupsDocument62 pagesAlkanes, Alkenes, Alkunas and Cycloalkanes: Discussion GroupsNing CahNo ratings yet
- Arihant Chemistry HandBookDocument14 pagesArihant Chemistry HandBookKannada SubjectNo ratings yet
- Non-Hydrocarbon - Esters: RCOOR Where R and R Represented The Same or Different Alkyl GroupsDocument2 pagesNon-Hydrocarbon - Esters: RCOOR Where R and R Represented The Same or Different Alkyl Groupscikgu ayuNo ratings yet
- Alcohol Phenol Ether and Carbonyl Compounds. Assignment Q. (Adv) .Document8 pagesAlcohol Phenol Ether and Carbonyl Compounds. Assignment Q. (Adv) .Anurag RamachandranNo ratings yet