Professional Documents
Culture Documents
Someunusualparaneoplasticsyndromes: Diagnosis in Oncology
Uploaded by
Mohamed Nuri ShembeshOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Someunusualparaneoplasticsyndromes: Diagnosis in Oncology
Uploaded by
Mohamed Nuri ShembeshCopyright:
Available Formats
DIAGNOSIS IN ONCOLOGY
Some Unusual Paraneoplastic Syndromes
CASE 1. METASTATIC SQUAMOUS CELL ESOPHAGEAL CANCER TO THE THUMB
A 56-year-old white male was received in our outpatient clinic because of slight pain and swelling on the thumb of his left hand. The patient reported a history of trauma with a shhook 3 days earlier but recognized that the nger was already swollen for a week. The symptoms were conned to the distal phalanx of the thumb, and on examination, it had an inammatory aspect (Fig 1). He had been diagnosed 3 months earlier with squamous cell esophageal carcinoma with metastatic supraclavicular lymph nodes. He was treated with palliative radiotherapy and cisplatin-uorouracil chemotherapy. A complete regression of the supraclavicular nodes and a resolution of the dysphagia were obtained. A radiograph of the thumb (Fig 2) showed destruction of the distal phalanx with soft-tissue tumor and lytic lesions in the proximal phalanx. A biopsy was performed, revealing metastatic squamous cell carcinoma. Recurrence of the esophageal tumor was detected by endoscopic examination, and it was the only evidence of disease relapse after work-up. The pain was controlled with nonsteroidal analgesics, but the patient developed recurrent dysphagia and started second-line chemotherapy. Bone metastases are frequent in cancer patients; however, acrometastases or metastases to the hand and foot are rare. Acrometastases have been reported in a variety of malignancies, such as breast, lung, gastrointestinal, and genitourinary tract, especially renal cell carcinoma.1 In some cases, it can be the rst manifestation of an occult cancer.2,3 A biopsy must be performed to conrm the diagnosis because many cases are often mistaken for benign processes, such as infection or inammatory arthritis. David Aguiar Bujanda, Uriel Bohn Sarmiento, and Jose Aguiar Morales Servicio de Oncologia Medica, Hospital de Gran Canaria Dr. Negrin, Las Palmas de Gran Canaria, Spain Copyright 2003 American Society of Clinical Oncology
Downloaded from jco.ascopubs.org on May 1, 2012. For personal use only. No other uses without permission. Copyright 2003 American Society of Clinical Oncology. All rights reserved.
Fig 1.
Inammation of the distal phalanx of the thumb.
Fig 2. A radiograph of the thumb showing destruction of the distal phalanx with soft-tissue tumor and lytic lesions in the proximal phalanx.
REFERENCES
1. Troncoso A, Ro JY, Grignon DJ, et al: Renal cell carcinoma with acrometastasis: Report of two cases and review of the literature. Mod Pathol 4:66-69, 1991 2. Janne PA, Datta MW, Johnson BE: Lung cancer presenting with solitary bone metastases. Case 2: Acrometastasis as an initial presentation of nonsmall-cell lung carcinoma. J Clin Oncol 17:2998-3001, 1999 3. Healey JH, Turnbull AD, Miedema B, et al: Acrometastases: A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am 68:743-746, 1986
EDITORS NOTE
See also Acrometastasis as an Initial Presentation of NonSmall-Cell Lung Carcinoma Janne P, et al: J Clin Oncol 17:2998-3001, 1999.
DOI: 10.1200/JCO.2003.06.074
CASE 2. DIGITAL ULCERATION AS A PARANEOPLASTIC SYNDROME IN OVARIAN CANCER
A 62-year-old woman presented in early November 1999 with a 12-week history of painful, pale ngers and toes of both hands and feet, worsening with exposure to cold, and cutaneous lesions on both hands. She also had an 8-week history of malaise,
2620
Journal of Clinical Oncology, Vol 21, No 13 (July 1), 2003: pp 2620-2625
DIAGNOSIS IN ONCOLOGY
2621
nausea, dizziness, diarrhea, unspecied weight loss, fatigue, and a periodically distended abdomen. There was no history of Raynauds phenomenon, vascular or connective tissue disease, or hyperlipidemia. Previous history was unremarkable. Physical examination revealed a pale, tired-looking afebrile woman with paronychia on digits two and four of both hands and feet and some bruising. Both the ngers and toes were pale, cool, and painful to touch. Peripheral pulses were present and symmetric. Livedo reticularis was visible on the ventral side of both legs. The abdomen was not distended or painful, and there was no shifting dullness. The physical examination was otherwise unremarkable. Laboratory results at that time were normal, except for mild thrombocytosis. In December 1999, while the analysis of dermal symptoms was still ongoing, she was diagnosed with pulmonary embolism arising from the deep venous thrombosis of the right femoral vein. Additional investigation, focused on malignancy, revealed a poorly differentiated adenocarcinoma of the right ovary, International Federation of Gynecology and Obstetrics classication stage IIIC, grade 3, which was subsequently treated with debulking surgery and six courses of chemotherapy. Evaluation of the skin lesions by immunouorescence for immunoglobulin (Ig) G, IgA, IgM, and complement C1q and C3c were negative, as were tests for autoimmune disease, cryoglobulins, and paraprotein. Magnetic resonance imaging of the hands did not show osseous abnormalities. Biopsy of the dermis revealed no epidermal abnormalities or signs of inammation, and there were no signs of vasculitis or tumor emboli. By exclusion of other possible diagnoses, we concluded that the skin condition was most likely a paraneoplastic syndrome. Treatment with nifedipine did not stop progression of the cutaneous symptoms, which deteriorated even though a clinical remission of the ovarian cancer was demonstrated after surgery and the rst courses of chemotherapy in March 2000 (Fig 1 and Fig 2, showing small necrotic ulcers with red indurated spots). It was then decided to start iloprost (Ilomedin; Schering AG, Berlin, Germany) 2 ng/kg over 6 hours intravenously for 5 days, with continuation of oral nifedipine. Instant clinical improvement was seen, with healing of the ulcers and disappearance of Raynaud complaints. Although iloprost treatment was administered for 5 days only, improvement continued for months, with almost complete disappearance of the dermatosis leaving only a few crusts on the proximal and distal interphalangeal joints and on the cuticles. The skin condition remained in remission until February 2001, when a mild relapse of symptoms occurred without clinical or biologic proof of tumor progression. The symptoms again responded to a short course of iloprost treatment. In May 2001, progression of the dermal symptoms coincided with tumor recurrence, and this time, the ulcers also appeared on the feet. The patient decided against palliative chemotherapy. Iloprost was administered, but this time, no effect was observed. The patient died from progressive ovarian cancer.
Downloaded from jco.ascopubs.org on May 1, 2012. For personal use only. No other uses without permission. Copyright 2003 American Society of Clinical Oncology. All rights reserved.
Fig 1. spots.
Photograph of patient showing small necrotic ulcers with red indurated
Fig 2. spots.
Photograph of patient showing small necrotic ulcers with red indurated
2622
AGRAWAL ET AL
Paraneoplastic dermal manifestations in ovarian cancer are uncommon.1,2 The physiopathology of most paraneoplastic syndromes remains elusive, and to date, no standard treatment has been dened. The distinct cutaneous paraneoplastic syndrome in our patient is extremely rare, and to our knowledge, only three similar cases have been reported.3-5 No specic diagnostic tools are available; therefore, the diagnosis of the dermatosis was conrmed by the clinical morphology of the lesions, by the temporal relationship with malignant disease, and by exclusion of other diagnoses.6 Unfortunately, biopsy is inconclusive,5 as was the case in our patient. Corticosteroids are mostly ineffective in these lesions,5 and therefore, in this patient, a symptom-directed policy was chosen with continuous intravenous infusion of the prostacyclin analog iloprost. Iloprost has vasodilating and platelet-inhibitory effects, and it is known to be effective in patients with peripheral arterial occlusive disease or with Raynauds phenomenon.7 Moreover, iloprost can produce signicant healing of digital ulcers.8 Treatment of a cutaneous paraneoplastic syndrome with iloprost has not been described before. It is of interest to note that, although treatment of the malignancy was not sufcient to induce a remission of the dermal manifestations in our patients, iloprost administration could not achieve improvement without treatment of the tumor. Wendela G. Pronk, Jan P. Baars, Paul Chr. de Jong, and Anneke M. Westermann Academic Medical Center, Amsterdam; Flevo Hospital, Almere; and St Anthonius Hospital, Nieuwegein, the Netherlands Copyright 2003 American Society of Clinical Oncology
REFERENCES
1. Ashour AA, Verschraegen CF, Kudelka AP, et al: Paraneoplastic syndromes of gynecologic neoplasms. J Clin Oncol 15:1272-1282, 1997 2. Sabir S, James WD, Schuchter LM: Cutaneous manifestations of cancer. Curr Opin Oncol 11:139-144, 1999 3. Hawley PR, Johnston AW, Rankin JT: Association between digital ischaemia and malignant disease. BMJ 3:208-212, 1967 4. Freundlich B, Makover D, Maul GG: A novel antinuclear antibody associated with a lupus-like paraneoplastic syndrome. Ann Intern Med 109:295-297, 1988 5. Chow SF, McKenna CH: Ovarian cancer and gangrene of the digits: Case report and review of the literature. Mayo Clin Proc 71:253-258, 1996 6. Cohen PR, Kurzrock R: Mucocutaneous paraneoplastic syndromes. Semin Oncol 24:334-359, 1997 7. Black CM, Halkier-Sorensen L, Belch JJF, et al: Oral iloprost in Raynauds phenomenon secondary to systemic sclerosis: A multicenter, placebo-controlled dose-comparison study. Br J Rheumatol 37:952-960, 1998 8. Wigley FM, Seibold JR, Wise RA, et al: Intravenous iloprost in the treatment of Raynauds phenomenon and ischaemic ulcers secondary to systemic sclerosis. J Rheumatol 19:1407-1414, 1992
Downloaded from jco.ascopubs.org on May 1, 2012. For personal use only. No other uses without permission. Copyright 2003 American Society of Clinical Oncology. All rights reserved.
EDITORS NOTE
See also Iamandi C, Dietemann A, Grosshans E, et al: Unusual presentations of lung cancer. Case 3: Paraneoplastic digital necrosis in a patient with small-cell lung cancer. J Clin Oncol 20:4600-4601, 2002
DOI: 10.1200/JCO.2003.07.099
CASE 3. METASTATIC PULMONARY CALCIFICATION CAUSING HYPOXEMIA IN MALE BREAST CANCER
A 56-year-old white male with previous left mastectomy for breast cancer and skeletal metastatic disease of a duration of 2 years was hospitalized with a history of nonproductive cough and dyspnea for 4 days. Physical examination revealed an afebrile, normotensive individual with a regular heart rate of 110 beats/min, respiratory rate of 20 beats/min, and fracture of the left tibia. Laboratory values were calcium 16.1 mg/dL, alkaline phosphatase 252 IU/L (normal 38 to 126 IU/L), creatinine 3.5 mg/dL, blood urea nitrogen 58 mg/dL, and phosphorus 4.8 mg/dL. Arterial blood gases on 40% oxygen by facemask showed a pH of 7.36, p02 of 46 mmHg, pC02 of 35 mmHg, HCO3 of 18 mEq/dL, and oxygen saturation of 80%. ECG showed sinus tachycardia. Chest radiograph (Fig 1) was normal except for blastic bone metastases on admission, showing, 48 hours later, diffuse bilateral pulmonary inltrates and consolidation with obliteration of the left heart border (Fig 2). Differential diagnosis included pulmonary edema, congestive heart failure, pneumonitis, lymphangitic metastases, and intrapulmonary hemorrhage. Two-dimensional echocardiogram showed a left-ventricular ejection fraction in the range of 25% and wall-motion abnormality consistent with coronary artery disease. Swan-Ganz catheterization showed pulmonary artery peak-systolic and end-diastolic pressures of 35 and 16 mmHg, respectively, and a pulmonary capillary wedge pressure of 12 mmHg, which was not consistent with a cardiac cause of pulmonary inltrates. Ventilation-perfusion nuclear study was unremarkable. The patient was initially treated with subcutaneous enoxaparin, intravenous imipenem, erythromycin, corticosteroids, calcitonin-salmon, and hemodialysis for oliguria. Although serum calcium, creatinine, and blood urea nitrogen slowly normalized, the diffuse pulmonary inltrates and dyspnea persisted. High-resolution computed tomography (HRCT; Fig 3) demonstrated several geographic areas of faint ground-glass density throughout both lungs, with focal areas of alveolar consolidation. Finally, bronchoscopic lung biopsy showed calcied lesions (Fig 4) of various shapes and sizes, primarily located in the thickened broproliferative interstitium of the lungs. Diffuse pulmonary calcication (also called metastatic calcication) will often have a benign etiology. It is most frequently encountered in chronic renal failure patients undergoing hemodialysis. Other causes, including hypervitaminosis D, primary
DIAGNOSIS IN ONCOLOGY
2623
Downloaded from jco.ascopubs.org on May 1, 2012. For personal use only. No other uses without permission. Copyright 2003 American Society of Clinical Oncology. All rights reserved.
Fig 1.
Chest radiograph of patient showing blastic bone metastases.
Fig 2. Chest radiograph showing diffuse bilateral pulmonary inltrates and consolidation with obliteration of the left heart border.
hyperparathyroidism, milk-alkali syndrome, and orthotopic hepatic transplantation, are much less common. Metastatic pulmonary calcication is occasionally associated with various malignancies. The underlying disturbance is an elevated serum calcium with or without an increased calcium-phosphate product. Hypercalcemia in a malignant disorder results from either skeletal metastases or humoral effects on the skeleton. Pulmonary calcication may lead to slowly diminishing diffusion capacity, and hypoxemia may ensue.1 In such cases, the patient may die from progressive respiratory failure.2,3 In other cases, the respiratory failure is rapid and rather acute.4,5 Two reported patients, one with B-cell lymphoma and the other with multiple myeloma, developed hypercalcemia and subsequent acute respiratory distress syndrome.5 Autopsy studies showed disseminated alveolar calcium deposition and diffuse alveolar damage. The standard chest x-ray is relatively insensitive to detect diffuse parenchymal pulmonary calcication. In addition, inltrates often cannot be distinguished from other intrapulmonary processes. Bone scintigraphy and HRCT scan with
Fig 3. High-resolution computed tomography demonstrating several geographic areas of faint ground-glass density throughout both lungs, with focal areas of alveolar consolidation.
Fig 4.
Bronchoscopic lung biopsy showing calcied lesions.
2624
EBERT, SHAFFER, AND RENNKE
mediastinal images are both superior to the standard chest x-ray in detecting pulmonary calcication. Pulmonary calcication is often misinterpreted as pneumonitis or pulmonary edema both on chest x-ray and computed tomography scan. Bone scintigraphy with bone-avid radiotracer 99mtechnetium-methylene diphosphate helps to resolve these equivocal cases. Twenty-four patients with positive pulmonary bone scintigraphy and hypercalcemia caused by hyperparathyroidism but without chest radiographic evidence of calcication have been described.6 A critical but often overlooked use of bone scintigraphy and HRCT is in the early and accurate recognition of pulmonary calcication in cases of unexplained and persistent inltrates. These tests further obviate the need for more invasive procedures, such as lung biopsy. In the right clinical setting, metastatic pulmonary calcication should be considered in the differential diagnosis of lung inltrates of both acute nature and, as in this case, chronic nature. Ravi S. Agrawal, Jaya R. Agrawal, Bharat L. Agrawal, Amaresh R. Nath, Asha R. Agrawal, J.C. Gaddipat and Richard F. Garnett, Jr Cancer and Blood Disease Center, and Department of Medicine, Reid Hospital & Health Care Services, Richmond, IN Copyright 2003 American Society of Clinical Oncology
REFERENCES
1. Khaf RA, DeLima C, Silverberg A, et al: Calciphylaxis and systemic calcinosis: Collective review. Arch Intern Med 150:956-959, 1990 2. McLachlan MSF, Wallace M, Seneviratne C: Pulmonary calcication in renal failure: Report of three cases. Br J Radiol 41:99-106, 1968 3. Davidson RC, Pendros JP: Calcium related cardio-respiratory death in chronic hemodialysis. Trans Am Soc Artif Intern Organs 13:36-40, 1967 4. Morii H, Ohnishi Y, Oko-moto T, et al: A case of B cell lymphoma complicated by hypercalcemia and acute respiratory insufciency. Osaka City Med J 29:75-85, 1983 5. Poe RH, Kamath C, Bauer MA, et al: Acute respiratory distress syndrome with pulmonary calcication in two patients with B cell malignancies. Respiration 56:127-133, 1989 6. Coolens JL, Devos P, De Roo M: Diffuse pulmonary uptake of 99m Tc bone-imaging agents: Case report and survey. Eur J Nucl Med 11:36-42, 1985 Downloaded from jco.ascopubs.org on May 1, 2012. For personal use only. No other uses without permission. Copyright 2003 American Society of Clinical Oncology. All rights reserved.
DOI: 10.1200/JCO.2003.09.104
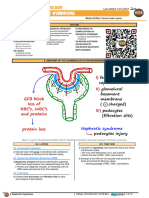
CASE 4. PARANEOPLASTIC NEPHROTIC SYNDROME IN A PATIENT WITH LUNG CANCER
A 69-year-old man with a 100 pack/year history of cigarette smoking was noted to have a lung mass on screening chest x-ray. A chest computed tomography (CT) revealed a 4 3 cm right lower-lobe (RLL) lung mass and a 1-cm precarinal node. Nonsmall-cell lung cancer was diagnosed by CT-guided biopsy of the pulmonary mass. A head CT and bone scan were negative for metastases. He was asymptomatic, and he chose to defer treatment of his lung cancer for 3 months until after his daughters marriage. Four months after diagnosis and before any therapy, he developed hemoptysis, worsening dyspnea, and bilateral lower-extremity edema. He noted that his urine had become extremely frothy. On physical examination, he had decreased breath sounds in the right lung base, abdominal distension, and 3 lower-extremity edema. A chest CT showed progression of his malignancy with RLL collapse, mediastinal adenopathy up to 3 cm in size, and a left upper-lobe nodule. Laboratory studies were signicant for a serum albumin of 2.3 mg/dL and a creatinine unchanged at 1.4 mg/dL. Urinalysis showed 3 protein, and a 24-hour urine collection contained 10.2 grams of protein. His serum cholesterol level was elevated at 308 mg/dL. A serum protein electrophoresis, antinuclear antibody (ANA), and renal ultrasound were unremarkable. He was treated with concomitant chemotherapy and radiation therapy consisting of 3,750 Gy of radiation to the RLL in 15 fractions, as well as weekly carboplatin at an area under the curve of 2 and taxol 50 mg/m2 for 5 weeks. Hemoptysis and dyspnea resolved in the rst month of treatment. Lower-extremity edema and abdominal distension improved gradually over 3 months. According to CT scans, his RLL mass decreased in size, and his RLL collapse resolved and remained stable over the subsequent 9 months (Fig 1). His blood pressure was controlled with lisinopril (Zestril; AstraZeneca, London, United Kingdom) and hydrochlorothiazide. His albumin increased to 3.7 gm/dL, his proteinuria decreased to 540 mg/24 hours, and his creatinine remained stable (Fig 2). Lung cancer is among the malignancies most commonly associated with a paraneoplastic nephrotic syndrome.1-3 Membranous nephropathy is most commonly associated with solid malignancies, whereas minimal change disease is seen most often in patients with Hodgkins disease.1,2,4 Presentation with nephrotic syndrome occurs before the diagnosis of cancer in approximately 40% of patients, at the time of diagnosis in 40% of patients, and after diagnosis in 20% of patients.2 Treatment of the neoplasm is associated with improvements in proteinuria.3,5 The pathogenesis of this paraneoplastic syndrome has been attributed to the production of cancer-related antigens, with subsequent damage to the glomerular basement membrane by antigen-antibody complexes.6 An alternative hypothesis is that an autoantibody, produced in response to the malignancy, may cross-react with and damage the glomerular basement membrane.
DIAGNOSIS IN ONCOLOGY
2625
Downloaded from jco.ascopubs.org on May 1, 2012. For personal use only. No other uses without permission. Copyright 2003 American Society of Clinical Oncology. All rights reserved.
Fig 1.
Computed tomography scan showing decreased right lower lobe mass. Fig 2. Graphs showing (A) 24-hour urine total protein, (B) serum albumin, and (C) serum creatinine.
Hypoalbuminemia is common in patients with lung cancer. A small subset of these patients will have nephrotic syndrome because of a paraneoplastic process. Benjamin Ebert, Kitt Shaffer, and Helmut Rennke Dana-Farber Cancer Institute, Brigham and Womens Hospital, and Harvard Medical School, Boston, MA Copyright 2003 American Society of Clinical Oncology
REFERENCES
1. Lee JC, Yamauchi H, Hopper J: The association of cancer and the nephrotic syndrome. Ann Intern Med 64:41-51, 1965 2. Burstein DM, Korbet SM, Schwartz MM: Membranous glomerulonephritis and malignancy. Am J Kidney Dis 22:5-10, 1993 3. Shikata Y, Hayashi Y, Yamazaki H, et al: Effectiveness of radiation therapy in nephrotic syndrome associated with advanced lung cancer. Nephron 83:160-164, 1999 4. Davison AM: Renal diseases associated with malignancies. Nephrol Dial Transplant 16:13-14, 2001 (suppl 6) 5. Boon ES, Vrij AA, Nieuwhof C, et al: Small cell lung cancer with paraneoplastic nephrotic syndrome. Eur Respir J 7:1192-1193, 1994 6. Costanza ME, Pinn V, Schwartz RS, et al: Carcinoembryonic antigenantibody complexes in a patient with colonic carcinoma and nephrotic syndrome. N Engl J Med 289:520-522, 1973
DOI: 10.1200/JCO.2003.11.021
You might also like
- Neuroendocrine Tumors: Surgical Evaluation and ManagementFrom EverandNeuroendocrine Tumors: Surgical Evaluation and ManagementJordan M. CloydNo ratings yet
- Ciliary Body Metastasis Masquerading As ScleritisDocument13 pagesCiliary Body Metastasis Masquerading As ScleritisDurgeshRastogiNo ratings yet
- Multiple Bilateral Choroidal Metastasis From Anal MelanomaDocument2 pagesMultiple Bilateral Choroidal Metastasis From Anal MelanomaMoazzam FarooqiNo ratings yet
- Esophageal Composite Tumor With Neuroendocrine Component: A Rare EntityDocument4 pagesEsophageal Composite Tumor With Neuroendocrine Component: A Rare EntityIJAR JOURNALNo ratings yet
- Letters To The Editors: Primary Sjögren's Syndrome As Paraneoplastic Disorder: A Case ReportDocument1 pageLetters To The Editors: Primary Sjögren's Syndrome As Paraneoplastic Disorder: A Case ReportDarian AngNo ratings yet
- Case report-PALDocument5 pagesCase report-PALHeshan SiriwardenaNo ratings yet
- Soft Tissue Swelling in Children: Case Report, Differential Diagnosis, and Diagnostic DelayDocument4 pagesSoft Tissue Swelling in Children: Case Report, Differential Diagnosis, and Diagnostic DelaywzlNo ratings yet
- Neuroendocrine Tumors Dr. WarsinggihDocument20 pagesNeuroendocrine Tumors Dr. WarsinggihAndi Eka Putra PerdanaNo ratings yet
- Krukenberg Tumour Simulating Uterine Fibroids and Pelvic Inflammatory DiseaseDocument3 pagesKrukenberg Tumour Simulating Uterine Fibroids and Pelvic Inflammatory DiseaseradianrendratukanNo ratings yet
- Dermatomyositis and Undifferentiated Nasopharyngeal Carcinoma. A Rare Presentation of A Rare MalignancyDocument4 pagesDermatomyositis and Undifferentiated Nasopharyngeal Carcinoma. A Rare Presentation of A Rare MalignancyasclepiuspdfsNo ratings yet
- Multiple Episodes of Hypoglycemia Secondary To An Insulinoma Case ReportDocument4 pagesMultiple Episodes of Hypoglycemia Secondary To An Insulinoma Case ReportmiguelNo ratings yet
- Case Report - Omental TorsionDocument9 pagesCase Report - Omental TorsionProf. M AmirNo ratings yet
- A Pain in The Butt - A Case Series of Gluteal Compartment Syndrome - PMCDocument6 pagesA Pain in The Butt - A Case Series of Gluteal Compartment Syndrome - PMCchhabraanNo ratings yet
- Case ReportDocument5 pagesCase ReportFerdina NidyasariNo ratings yet
- Early - Stage Hodgkins Lymphoma in A Child A CaseDocument6 pagesEarly - Stage Hodgkins Lymphoma in A Child A CaseХарш Навнітбхаі НімаватNo ratings yet
- Malignant Mesothelioma Presenting With Unexplained Recurrent Pleurisy EpisodesDocument4 pagesMalignant Mesothelioma Presenting With Unexplained Recurrent Pleurisy EpisodesNurul NingrumNo ratings yet
- Appendiceal Mucinous Adenocarcinoma With Concurrent Tuberculous Appendix: An Unusual Overlap of Two Rare DiseasesDocument1 pageAppendiceal Mucinous Adenocarcinoma With Concurrent Tuberculous Appendix: An Unusual Overlap of Two Rare DiseasesKhei Jazzle LimNo ratings yet
- Ajol File Journals - 76 - Articles - 114038 - Submission - Proof - 114038 901 318286 1 10 20150309Document2 pagesAjol File Journals - 76 - Articles - 114038 - Submission - Proof - 114038 901 318286 1 10 20150309alysNo ratings yet
- Non-Healing Diabetic Ulcer TreatedDocument11 pagesNon-Healing Diabetic Ulcer TreatedKristie AndersonNo ratings yet
- Hon 2005Document2 pagesHon 2005Andana TrisaviNo ratings yet
- Scurvy Masquerading As Juvenile Idiopathic Arthritis or VasculitisDocument6 pagesScurvy Masquerading As Juvenile Idiopathic Arthritis or VasculitisZach Segmuel MiñanoNo ratings yet
- Paraneoplastic Dermatomyositis Revealing Ovarian Cancer: About A CaseDocument5 pagesParaneoplastic Dermatomyositis Revealing Ovarian Cancer: About A CaseIJAR JOURNALNo ratings yet
- Adult Intussussception Int J Student Res 2012Document3 pagesAdult Intussussception Int J Student Res 2012Juan De Dios Diaz-RosalesNo ratings yet
- Revoke SuretyDocument3 pagesRevoke Suretysonya.rylee99No ratings yet
- Dgab 512Document23 pagesDgab 512henry hernandezNo ratings yet
- Incidentalomas SuparrenalesDocument23 pagesIncidentalomas SuparrenalesAlexandra FreireNo ratings yet
- Eyelid TattoingDocument15 pagesEyelid Tattoing小島隆司No ratings yet
- 48 Devadass EtalDocument4 pages48 Devadass EtaleditorijmrhsNo ratings yet
- Dgab 512Document23 pagesDgab 512adri20121989No ratings yet
- Case On Ankle SurgeryDocument13 pagesCase On Ankle SurgeryLuqman AhmedNo ratings yet
- OvaryDocument4 pagesOvaryCarolina MartinezNo ratings yet
- Lucivero Et Al 2011 Lupus Mastitis in Systemic Lupus Erythematosus A Rare Condition Requiring A Minimally InvasiveDocument5 pagesLucivero Et Al 2011 Lupus Mastitis in Systemic Lupus Erythematosus A Rare Condition Requiring A Minimally Invasiveruthdaniel6041No ratings yet
- BPJ Vol 10 No 3 P 1369-1377Document9 pagesBPJ Vol 10 No 3 P 1369-1377citra annisa fitriNo ratings yet
- 18 Extra-GuptaNDocument3 pages18 Extra-GuptaNGalih rarang gatiNo ratings yet
- Calciphylaxis, Another Facet of Mastitis: A Case ReportDocument5 pagesCalciphylaxis, Another Facet of Mastitis: A Case ReportIJAR JOURNALNo ratings yet
- Pi Is 1542356517303877Document2 pagesPi Is 1542356517303877riski novitaNo ratings yet
- Junal PyodermaDocument6 pagesJunal PyodermaJares Clinton Saragih SimarmataNo ratings yet
- Ijerph 18 05895Document13 pagesIjerph 18 05895JHon Hendrik TambunanNo ratings yet
- Endoscopia: Diagnostic Approach of Intestinal Tuberculosis: Case and Literature ReviewDocument4 pagesEndoscopia: Diagnostic Approach of Intestinal Tuberculosis: Case and Literature ReviewDumitru RadulescuNo ratings yet
- Kurtz 2016Document5 pagesKurtz 2016Stevano PattiasinaNo ratings yet
- 160-Article Text-621-2-10-20221125Document6 pages160-Article Text-621-2-10-20221125Harmanjot SinghNo ratings yet
- Ultrasonography: A Cost - Effective Modality For Diagnosis of Rib Tuberculosis - A Case ReportDocument3 pagesUltrasonography: A Cost - Effective Modality For Diagnosis of Rib Tuberculosis - A Case ReportAdvanced Research PublicationsNo ratings yet
- Mediastinal Mass in A 25-Year-Old Man: Chest Imaging and Pathology For CliniciansDocument5 pagesMediastinal Mass in A 25-Year-Old Man: Chest Imaging and Pathology For CliniciansWahyu RianiNo ratings yet
- Comninos 2019Document4 pagesComninos 2019Priscilla GeraldineNo ratings yet
- The Use of Traditional Chinese Veterinary Medicine in The Treatment of 5 Cases of Neoplastic Bone DiseaseDocument12 pagesThe Use of Traditional Chinese Veterinary Medicine in The Treatment of 5 Cases of Neoplastic Bone DiseaseDonnaNo ratings yet
- Primary Splenic Amyloidosis: A Case ReportDocument4 pagesPrimary Splenic Amyloidosis: A Case Reportfitra khoNo ratings yet
- Masquerade SyndromesDocument10 pagesMasquerade Syndromestony_chrisNo ratings yet
- Cutaneous Polyarteritis Nodosa in A Child FollowinDocument2 pagesCutaneous Polyarteritis Nodosa in A Child FollowinandresrogersNo ratings yet
- Primary Umbilical Endometriosis. Case Report and Discussion On Management OptionsDocument7 pagesPrimary Umbilical Endometriosis. Case Report and Discussion On Management Optionsari naNo ratings yet
- Urology Case Reports: Daniel Ensley, Grant H. EvansDocument2 pagesUrology Case Reports: Daniel Ensley, Grant H. EvansFrankenstein MelancholyNo ratings yet
- Iannetti 2010Document4 pagesIannetti 2010FrancescFranquesaNo ratings yet
- Amyotrophic Dermatomyositis PDFDocument1 pageAmyotrophic Dermatomyositis PDFM.DalaniNo ratings yet
- Case ReportDocument6 pagesCase ReportYerly Ramirez MuñozNo ratings yet
- Medicine: Systemic Lupus Erythematosus With Guillian - Barre SyndromeDocument5 pagesMedicine: Systemic Lupus Erythematosus With Guillian - Barre SyndromeShri RamNo ratings yet
- Case Report: Vertigo As A Predominant Manifestation of NeurosarcoidosisDocument5 pagesCase Report: Vertigo As A Predominant Manifestation of NeurosarcoidosisDjumadi AkbarNo ratings yet
- Necrotisans Erythema Nodosum Leprosum With Systemic ManifestDocument1 pageNecrotisans Erythema Nodosum Leprosum With Systemic Manifestzak bearNo ratings yet
- Ovarian TorsionDocument4 pagesOvarian TorsionRiko KuswaraNo ratings yet
- Ovarian Myeloid Sarcoma, Gynecol Oncol Rep 2023Document7 pagesOvarian Myeloid Sarcoma, Gynecol Oncol Rep 2023Semir VranicNo ratings yet
- 6703 23736 1 PBDocument3 pages6703 23736 1 PBReski Harlianty HarliNo ratings yet
- Lethal Midline Granuloma Importance of Early Diagnosis: A Case ReportDocument0 pagesLethal Midline Granuloma Importance of Early Diagnosis: A Case ReportdeolukmanaNo ratings yet
- Sindrom NefrotikDocument31 pagesSindrom NefrotikMohammed Ramzy GhifariNo ratings yet
- Vomiting in A Neonate Dorothy Damore MDDocument5 pagesVomiting in A Neonate Dorothy Damore MDHanny RusliNo ratings yet
- HESI Exam PracticeDocument33 pagesHESI Exam PracticeJean AustenNo ratings yet
- Nursing Care Plan Nephrotic SyndromeDocument5 pagesNursing Care Plan Nephrotic SyndromeJhusmin BambicoNo ratings yet
- Nephrotic Syndrome - Armando HasudunganDocument18 pagesNephrotic Syndrome - Armando HasudunganzahraaNo ratings yet
- 1 Childhood Nephrotic Syndrome - Diagnosis and ManagementDocument52 pages1 Childhood Nephrotic Syndrome - Diagnosis and ManagementThana Balan100% (1)
- Nephrotic Syndrome in ChildrenDocument29 pagesNephrotic Syndrome in ChildrenFreeburn SimunchembuNo ratings yet
- Kode Icd X & Icd Ix CMDocument659 pagesKode Icd X & Icd Ix CMRichard SiahaanNo ratings yet
- Progress Test Sp1 23 Maret 2022: Kolegium IKA IndonesiaDocument31 pagesProgress Test Sp1 23 Maret 2022: Kolegium IKA IndonesiafiraNo ratings yet
- Mercury Contamination in Water & Its Impact On Public HealthDocument8 pagesMercury Contamination in Water & Its Impact On Public HealthHerman KarimNo ratings yet
- Nephrotic Syndrome in Adults: Acute Medicine 2018 17 (1) : 36-43 36Document8 pagesNephrotic Syndrome in Adults: Acute Medicine 2018 17 (1) : 36-43 36Deddy TriwijayaNo ratings yet
- Renal Pathology - 012) Nephrotic Syndrome (Notes)Document10 pagesRenal Pathology - 012) Nephrotic Syndrome (Notes)hasanatiya41No ratings yet
- KDIGO 2021 Glomerular Diseases Guideline (137 272)Document136 pagesKDIGO 2021 Glomerular Diseases Guideline (137 272)Kevin Lopez MartinezNo ratings yet
- Medicine All DIagnosis by AVI SeriesDocument8 pagesMedicine All DIagnosis by AVI SeriesMuhammad Awais Hassan AwaanNo ratings yet
- Nephrotic and Nephritic Syndrome: Med5010 LectureDocument65 pagesNephrotic and Nephritic Syndrome: Med5010 LectureFreeburn Simunchembu100% (1)
- HematuriaDocument42 pagesHematuriaWasim R. IssaNo ratings yet
- Pathology of Common Glomerular Syndromes: DR Purushotham KrishnappaDocument34 pagesPathology of Common Glomerular Syndromes: DR Purushotham KrishnappaTarin IslamNo ratings yet
- Pathophysiology Q's 1Document68 pagesPathophysiology Q's 1alibel_belloNo ratings yet
- Nursing Practice TestDocument19 pagesNursing Practice TestEdward Nicko GarciaNo ratings yet
- Natural Medicine Kidney DiseaseDocument36 pagesNatural Medicine Kidney Diseaselpdb3171No ratings yet
- Anatomy WordDocument86 pagesAnatomy WordYana-Marisa EdwardsNo ratings yet
- RENAL DISEASES Lab Act No. 22Document32 pagesRENAL DISEASES Lab Act No. 22KimberlyjoycsolomonNo ratings yet
- Paediatrics SbaDocument34 pagesPaediatrics SbaAhmad Syahmi YZ100% (1)
- Nephrotic SyndromeDocument17 pagesNephrotic SyndromeCitra Wulandari SofyanNo ratings yet
- Daftar Pustaka FixDocument2 pagesDaftar Pustaka FixAlmira Shabrina SaraswatiNo ratings yet
- Nephrotic SyndromeDocument57 pagesNephrotic SyndromePradnya WartheNo ratings yet
- Viteri2018 Prevalensi 5Document17 pagesViteri2018 Prevalensi 5adrian revoNo ratings yet
- Nephrotic Syndrome - NotesDocument22 pagesNephrotic Syndrome - NotesHampson Malekano100% (1)
- Renal DiseasesDocument26 pagesRenal DiseasesismailcemNo ratings yet
- MS 16Document7 pagesMS 16Ferdinand TerceroNo ratings yet