Professional Documents
Culture Documents
Flow Cytometr1
Uploaded by
vijendOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Flow Cytometr1
Uploaded by
vijendCopyright:
Available Formats
EXPERIMENT NOFLOW CYTOMETRY
AIM
FLOW CYTOMETRY: Flow cytometry is a technique for counting, examining, and sorting microcopy particles suspended in stream of fluid. It allows simultaneous analysis of the physical and/or chemical characteristic of single cells flowing through an optical ~nd/c)r electronic detection apparatus Thc first fluresence based flow cytometry device (ICP I 1) was developed in the year 1968 by Wolfgang Gohde from the University of Punsters Germany and first commercialized in l968/69 by German developer and manufacturer Partec through Phywe AG in Gottingen. The original name of the flow cytometry technology was pulse cytophotometry PRINCIPLE A beam of light (usually laser light) of a single wavelength is directed onto a hydrodynamically focused stream of fluid. A number of detectors are aimed at the point where the stream passes through the light beam; one in line with the light beam (Forward Scatter or FSC) and several perpendicular to it (Side Scatter (SSC) and one or more fluorescent detectors). Each suspended particle from 0.2 to 150 micrometers passing through the beam scatters the light in some way, and fluorescent chemicals found in the particle or attached to the particle may be excited into emitting light at a higher wavelength than the light source. This combination of scattered and fluorescent light is picked up by the detectors, and by analyzing fluctuations in brightness at each detector (one for each fluorescent emission peak) it is then possible to derive various types of information about the physical and chemical structure of each individual particle. FSC correlates with the cell volume and SSC depends on the inner complexity of the particle (i.e. shape of the nucleus, the amount and type of cytoplasmic granules or the membrane roughness). Soma flow cytomcters on the market have eliminated the need for fluorescence and use only light scarer for measurement. Other flow cytometcrs form images of each cell's fluorescence, Caned light, and transmitted light. FLOW CYTOMETERS: Modern flow cytometers are able to analyze several thousand particles every second, in "real time", and can actively separate and isolate particles having specified properties. A flow cytometer is similar to a microscope, except that instead of producing an image of the cell, flow cytometry offers "high-throughput" (for a large number of cells) automated quantification of set parameters. To analyze solid tissues single-cell suspension must first be prepared.
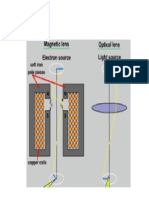
A flow cytometer has 5 main components: 1 . A flow cell - liquid stream (sheath fluids ca^l^Ties and aligns the cells so that they pass single file through the light beam for sensing. 2. A optical system - commonly used arc lamps (mercury, xenon~; high power water-cooled lasers (argon, krypton, dye laser); low power air cooled lasers argon 488nm,red HeNe 633nm,green-HeNe (UV),diode lasers resulting in light signals. 3. A detector & Analogue to Digital conversion(ADC) system-generating FSC & SSC as well as florescence signals from light into electrical signals that can be processed by computer. 4. An amplification system linear or logarithmic 5.A computer for analysis of the signals. Modern instruments usually have multiple lasers and fluorescence detectors (the current for a commercial instrument is 4 lasers and 18 fluorescence detectors). Increasing the number of layers & detectors allows for multiple antibody labelling and can identify a Orgy population by their phenotype. Certain instruments can cozen d4bl images of individual cells, allowing for tile analysis of fluoresccnt signal location within or on surface of cell The data generated by flow cytometers can be plotted in a single dimension, two dimensional dot plots or even in three dimensions. The regions on these plots can be sequentially separated, based on fluorescence intensity, by creating series of subset extractions, termed Gates. Specific gating protocols exist for diagnostic. Fluorescence-activated cell sorting (FACS) Fluorescence-activated cell sorting (FACS) is a specialized type of flow cytometry. It provides a method for sorting a heterogeneous mixture of biological cells into two or more containers, one cell at a time, based upon the specific light scattering florescent characteristics of each cell. It is a useful scientific instrument, as it provides fast, objective and quantitative recording of fluorescent signals from individual cells as well as physical separation of cells of particular interest. Among the large majority of researchers who use this technology for sorting or analysis, this term has become generic in common usage, much like xerox. The first cell sorter was invented by Mack Fulwyler in 1965, a relatively difficult technique and one no longer used in modern instruments. The cell suspension is entrained in the center of a narrow, rapidly flowing stream of liquid. The flow is arranged so that there is a large separation between cells relative to their diameter. A vibrating mechanism causes the stream of cells to break into individual droplets. The system is adjusted so that there is a low probability of more than one cell per droplet. Just before the stream breaks into droplets, the flow passes through a fluorescence measuring station where the fluorescent character of interest of each cell is measured. An electrical charging ring is placed just at the point where the stream breaks into droplets. A charge is placed on the ring based on the immediately prior fluorescence intensity measurement, and the opposite charge is trapped on the droplet as it breaks from the stream.
The charged droplets then fall through an electrostatic deflection system that diverts droplets into containers based upon their charge. In some systems, the charge is applied directly to the stream, and the droplet breaking off retains charge of the same sign as the stream. The stream is then returned to neutral after the droplet breaks off. Labels: 1. Fluorescent labels A wide range of fluorophores can be used as labels in flow cytometry. Fluorophores, or simply "fluors", are typically attached to an antibody that recognises a target feature on or in the cell; they may also be attached to a chemical entity with affinity for the cellular structure. Each fluorophore has a characteristic peak wavelength, and the emission spectra often overlap. Consequently, the combination of labels which can be used depends on the wavelength of the lamp(s) or laser(s) used to excite the fluorochromes and on the detectors available. The maximum number of distinguishable fluorescent labels is thought to be 17 or 18, and this level of complexity necessitates laborious optimization to limit artifacts, as well as complex algorithms to separate overlapping spectra. 2. Quantum dots sometimes used in place of traditional fluorophores because of their narrower emission peaks. 3. Isotope labeling In one approach to overcoming the fluorescent labeling limit, isotopes are attached to antibodies. This method could theoretically allow the use of 40 to 60 distinguishable labels and has been demonstrated for 30 labels. Cells are introduced into, ionizing them and allowing to identify the associated isotopes. Although this method permits the use of a large number of labels, it currently has lower throughput capacity than traditional flow cytometry. It also destroys the analysed cells, precluding their recovery by sorting. Measurable parameters This list is very long and constantly expanding. volume and complexity of cells cell pigment sorting (library construction, chromosome paint) protein expression and localization, Protein modifications, phospho-proteins transgenic products in vivo, particularly the Fluorescent Proteins cell surface (CD) markers) antigens (secondary mediators, etc.) enzymatic activity quantification, measurement of DNA degradation, mitochondrial membrane potential, permeability changes,) cell viability monitoring electropermeabilization of cells characterising (MDR) in cancer cells various combinations (DNA/surface antigens, etc.) cell adherence (for instance pathogen-host cell adherence)
You might also like
- Complete Basic Flow Cytometry Principles ManualDocument37 pagesComplete Basic Flow Cytometry Principles ManualcandiddreamsNo ratings yet
- "Next Friend" and "Guardian Ad Litem" - Difference BetweenDocument1 page"Next Friend" and "Guardian Ad Litem" - Difference BetweenTeh Hong Xhe100% (2)
- FlowcytometryDocument84 pagesFlowcytometryyourinmyheart100% (1)
- Flow Cytometry Basic TrainingDocument56 pagesFlow Cytometry Basic TrainingLinguumNo ratings yet
- Flow Cytometry: By: Dr. Megha Gupta & Dr. Tashi AgarwalDocument116 pagesFlow Cytometry: By: Dr. Megha Gupta & Dr. Tashi Agarwalanggaririn100% (1)
- Alugbati Plant Pigment Extraction As Natural Watercolor SourceDocument6 pagesAlugbati Plant Pigment Extraction As Natural Watercolor SourceMike Arvin Serrano100% (1)
- Fluorescence Microscopy: Super-Resolution and other Novel TechniquesFrom EverandFluorescence Microscopy: Super-Resolution and other Novel TechniquesAnda CorneaNo ratings yet
- Production of Alcohol Lecture NotesDocument3 pagesProduction of Alcohol Lecture Notesvijend100% (4)
- Flow Cytometry Basic TrainingDocument91 pagesFlow Cytometry Basic TrainingSupriya Kumar Das100% (2)
- Bacterial Genome Assembly IlluminaDocument49 pagesBacterial Genome Assembly IlluminadksaNo ratings yet
- Pe 3 Syllabus - GymnasticsDocument7 pagesPe 3 Syllabus - GymnasticsLOUISE DOROTHY PARAISO100% (1)
- Case Study of Milk ProductionDocument46 pagesCase Study of Milk Productionmian21100% (2)
- Notes On LC-MSDocument6 pagesNotes On LC-MSAlabhya DasNo ratings yet
- Flowcytometry 150403122734 Conversion Gate01 PDFDocument116 pagesFlowcytometry 150403122734 Conversion Gate01 PDFsachindra kumar100% (1)
- Plant Nutrition NotesDocument4 pagesPlant Nutrition Notesvijend100% (1)
- Standards Spec Brochure ME WEBDocument44 pagesStandards Spec Brochure ME WEBReza TambaNo ratings yet
- Flow Cytometry: Meroj A. JasemDocument58 pagesFlow Cytometry: Meroj A. Jasemahmad100% (1)
- A Beginners Guide To Flow Cytometry PDFDocument8 pagesA Beginners Guide To Flow Cytometry PDFBrahmananda Chakraborty100% (1)
- Principles, Applications and InterpretationsDocument64 pagesPrinciples, Applications and InterpretationsAbbi Yanto ArtNo ratings yet
- Flow CytometryDocument6 pagesFlow CytometryOCCULTEDNo ratings yet
- Fluorescence-Activated Cell SortingDocument10 pagesFluorescence-Activated Cell SortingShaher Bano MirzaNo ratings yet
- Introduction to Electrophysiological Methods and InstrumentationFrom EverandIntroduction to Electrophysiological Methods and InstrumentationNo ratings yet
- Flow CytometryDocument9 pagesFlow CytometryShown RichardsNo ratings yet
- FACSDocument8 pagesFACSKarthick ThiyagarajanNo ratings yet
- CytometryDocument11 pagesCytometrysachinpardeshiNo ratings yet
- FACSDocument24 pagesFACSMudit MisraNo ratings yet
- Flow CytometeryDocument26 pagesFlow CytometeryAmirNo ratings yet
- Flow CytometryDocument35 pagesFlow CytometryAdwika DeoNo ratings yet
- Introduction To Flow Cytometry May 10Document7 pagesIntroduction To Flow Cytometry May 10A_ArunyajNo ratings yet
- Fluorescence Activated Cell Sorter: Sudhanshu Shekhar M.Tech (Biotech) III Sem A7110709009Document24 pagesFluorescence Activated Cell Sorter: Sudhanshu Shekhar M.Tech (Biotech) III Sem A7110709009Sudhanshu ShekharNo ratings yet
- Principle of Flow CytometryDocument8 pagesPrinciple of Flow CytometrySteve D'HamsNo ratings yet
- Fluorescence Activated Cell SortingDocument5 pagesFluorescence Activated Cell Sortingsiya sharmaNo ratings yet
- Flow CytometryDocument7 pagesFlow CytometryWNo ratings yet
- What Is Flow CytometryDocument8 pagesWhat Is Flow CytometryRuksanurNo ratings yet
- Flow CytometryDocument13 pagesFlow CytometryNTA UGC-NETNo ratings yet
- Flow CytometryDocument7 pagesFlow CytometryAbhishek KumarNo ratings yet
- Flourscence Activated Cell SortingDocument6 pagesFlourscence Activated Cell SortingSalman KhanNo ratings yet
- Flowcytometry: Dr. Noune Ahs CihsrDocument30 pagesFlowcytometry: Dr. Noune Ahs CihsrAniapthingNo ratings yet
- Flow CytometryDocument6 pagesFlow Cytometrytanweer_elevenNo ratings yet
- Introduction To Flow Cytometry May 10 PDFDocument7 pagesIntroduction To Flow Cytometry May 10 PDFSilviaGonzalezSierraNo ratings yet
- (Doi 10.1002 - Cpim.40) Coligan, John E. Bierer, Barbara E. Margulies, David H. Sheva - Current Protocols in Immunology - Flow Cytometry - An OverviewDocument11 pages(Doi 10.1002 - Cpim.40) Coligan, John E. Bierer, Barbara E. Margulies, David H. Sheva - Current Protocols in Immunology - Flow Cytometry - An OverviewMunir AliNo ratings yet
- Flow Cytometry - An OverviewDocument16 pagesFlow Cytometry - An OverviewMariana BarretoNo ratings yet
- Flow Cytometry WriteupDocument3 pagesFlow Cytometry Writeupsuman palNo ratings yet
- Flowcytometry 190816150436Document20 pagesFlowcytometry 190816150436AmandaNo ratings yet
- Flow Cytometry Past and FutureDocument11 pagesFlow Cytometry Past and Futurececy loNo ratings yet
- 824-Texto Del Manuscrito Completo (Cuadros y Figuras Insertos) - 4381-1!10!20120828Document6 pages824-Texto Del Manuscrito Completo (Cuadros y Figuras Insertos) - 4381-1!10!20120828Tallie ZeidlerNo ratings yet
- Day 5 Serology LectureDocument52 pagesDay 5 Serology LectureMakuach Gatluak YakNo ratings yet
- What Light Source Is Used in Most Flow CytometersDocument4 pagesWhat Light Source Is Used in Most Flow CytometersMariel AbatayoNo ratings yet
- Flow CytometryDocument25 pagesFlow CytometryHarminderNo ratings yet
- Practical Flow Cytometry - M.Sc. 2nd Sem Immuno - FinalDocument5 pagesPractical Flow Cytometry - M.Sc. 2nd Sem Immuno - FinalAnusua RoyNo ratings yet
- Analytical Techniques in Biotechnology LABORATORY (BT1403) : R.Aruna Vigneshwari 96507214001Document27 pagesAnalytical Techniques in Biotechnology LABORATORY (BT1403) : R.Aruna Vigneshwari 96507214001Aruna VigneshwariNo ratings yet
- Bojan: MultiparameterDocument12 pagesBojan: MultiparameterMekaTronNo ratings yet
- Immunologhy Lab Report 3 - MURAT GENÇ-2021652244Document10 pagesImmunologhy Lab Report 3 - MURAT GENÇ-2021652244Murat GençNo ratings yet
- Flow Cytometry Facs ServicesDocument3 pagesFlow Cytometry Facs ServicesSteven4654No ratings yet
- Research Paper On Flow CytometryDocument7 pagesResearch Paper On Flow Cytometryafmcwqkpa100% (1)
- Adan2017 Flow Citometry Basic Principles and ApplicationsDocument15 pagesAdan2017 Flow Citometry Basic Principles and ApplicationsEdgar Velastegui GonzálezNo ratings yet
- SG 8 CH 15 InstrumentationDocument5 pagesSG 8 CH 15 InstrumentationwerfsdsfNo ratings yet
- Flow Cytometry Introduction - AbcamDocument11 pagesFlow Cytometry Introduction - AbcamMaitree UpadhayayNo ratings yet
- Principles of Flow CytometryDocument6 pagesPrinciples of Flow CytometryMohammad Abul FarahNo ratings yet
- Flow CytometryDocument3 pagesFlow CytometrySadananda KumbhakarNo ratings yet
- Introduction To FlowcytometerDocument11 pagesIntroduction To FlowcytometerSooridarsan KrishnanNo ratings yet
- Shapiro 1983 CytometryDocument17 pagesShapiro 1983 CytometryMunir AliNo ratings yet
- Flow Cytometry Basic Principles and ApplicationsDocument15 pagesFlow Cytometry Basic Principles and ApplicationsJoiceRodolphoNo ratings yet
- Quiz2 NoteseditDocument33 pagesQuiz2 NoteseditEricka GenoveNo ratings yet
- Table of ContentDocument11 pagesTable of ContentNaz AliNo ratings yet
- SpectrophotometryDocument22 pagesSpectrophotometryaziskfNo ratings yet
- Explain About Flourocytometry.Document4 pagesExplain About Flourocytometry.mandolinjobNo ratings yet
- Equ28-01 Sysmex XE2100 Op SOPDocument16 pagesEqu28-01 Sysmex XE2100 Op SOPWasim AkramNo ratings yet
- Promoters Used in Vector Construction For Genetic Engineering in Plants S.Y. Botany - IIIDocument2 pagesPromoters Used in Vector Construction For Genetic Engineering in Plants S.Y. Botany - IIIvijendNo ratings yet
- FY Transport of WaterDocument4 pagesFY Transport of WatervijendNo ratings yet
- Ecosystem Structure FY LectureDocument24 pagesEcosystem Structure FY LecturevijendNo ratings yet
- Ecosystems StructureDocument4 pagesEcosystems StructurevijendNo ratings yet
- Practical Exercise No. Notes. V.P.S. Shekhawat Cell Inclusions: Starch Grains (Potato and Rice) Aleurone Layer (Maize)Document5 pagesPractical Exercise No. Notes. V.P.S. Shekhawat Cell Inclusions: Starch Grains (Potato and Rice) Aleurone Layer (Maize)vijendNo ratings yet
- Starch ProcedureDocument1 pageStarch ProcedurevijendNo ratings yet
- EcosystemsDocument4 pagesEcosystemsvijendNo ratings yet
- SY CarbohydratesDocument5 pagesSY CarbohydratesvijendNo ratings yet
- What Is Somatic Embryogenesis? Discuss Important Phases, Factors Affecting and Various Applications of Somatic EmbryogenesisDocument1 pageWhat Is Somatic Embryogenesis? Discuss Important Phases, Factors Affecting and Various Applications of Somatic EmbryogenesisvijendNo ratings yet
- Practical FermentationDocument4 pagesPractical FermentationvijendNo ratings yet
- TerrariumDocument3 pagesTerrariumvijendNo ratings yet
- Alerone LayerDocument11 pagesAlerone LayervijendNo ratings yet
- Plant Tissue Culture MediaDocument34 pagesPlant Tissue Culture MediavijendNo ratings yet
- Lens Electron MicroscopeDocument1 pageLens Electron MicroscopevijendNo ratings yet
- SY Culture MediaDocument2 pagesSY Culture MediavijendNo ratings yet
- Promoters Used in Vector Construction For Genetic Engineering in Plants S.Y. Botany - IIIDocument2 pagesPromoters Used in Vector Construction For Genetic Engineering in Plants S.Y. Botany - IIIvijendNo ratings yet
- M.Sc.I CAMDocument22 pagesM.Sc.I CAMvijendNo ratings yet
- FY PhytoremediationDocument11 pagesFY PhytoremediationvijendNo ratings yet
- Ty PTC MediaDocument2 pagesTy PTC MediavijendNo ratings yet
- SY B.sc. Electromagnetic SpectrumDocument4 pagesSY B.sc. Electromagnetic SpectrumvijendNo ratings yet
- Cloning VectorsDocument27 pagesCloning VectorsvijendNo ratings yet
- Bioremediation Strategies LecturesDocument1 pageBioremediation Strategies LecturesvijendNo ratings yet
- Koi Deewana Kehta HainDocument3 pagesKoi Deewana Kehta HainvijendNo ratings yet
- T.Y. B.Sc. LiposomesDocument3 pagesT.Y. B.Sc. LiposomesvijendNo ratings yet
- FY Lecture Notes BiodiversityDocument2 pagesFY Lecture Notes Biodiversityvijend100% (2)
- Ecosystem ProductivityDocument2 pagesEcosystem ProductivityvijendNo ratings yet
- FY Lecture Notes BiodiversityDocument2 pagesFY Lecture Notes Biodiversityvijend100% (2)
- FY Lecture Notes BiodiversityDocument2 pagesFY Lecture Notes Biodiversityvijend100% (2)
- Recruitement Process - Siemens - Sneha Waman Kadam S200030047 PDFDocument7 pagesRecruitement Process - Siemens - Sneha Waman Kadam S200030047 PDFSneha KadamNo ratings yet
- 7 UpDocument3 pages7 UpRajeev TripathiNo ratings yet
- READING 4 UNIT 8 Crime-Nurse Jorge MonarDocument3 pagesREADING 4 UNIT 8 Crime-Nurse Jorge MonarJORGE ALEXANDER MONAR BARRAGANNo ratings yet
- Chapter Three Liquid Piping SystemDocument51 pagesChapter Three Liquid Piping SystemMelaku TamiratNo ratings yet
- Mainstreaming Gad Budget in The SDPDocument14 pagesMainstreaming Gad Budget in The SDPprecillaugartehalagoNo ratings yet
- PMI Framework Processes PresentationDocument17 pagesPMI Framework Processes PresentationAakash BhatiaNo ratings yet
- Minyak Atsiri Sereh WangiDocument4 pagesMinyak Atsiri Sereh Wangicindy paraditha kasandraNo ratings yet
- Chapter 2 and 3 ImmunologyDocument16 pagesChapter 2 and 3 ImmunologyRevathyNo ratings yet
- Oil ShaleDocument13 pagesOil Shalergopi_83No ratings yet
- DOWSIL™ 2-9034 Emulsion: Features & BenefitsDocument5 pagesDOWSIL™ 2-9034 Emulsion: Features & BenefitsLaban KantorNo ratings yet
- S ELITE Nina Authors Certain Ivey This Reproduce Western Material Management Gupta Names Do OntarioDocument15 pagesS ELITE Nina Authors Certain Ivey This Reproduce Western Material Management Gupta Names Do Ontariocarlos menaNo ratings yet
- Quality Factor of Inductor and CapacitorDocument4 pagesQuality Factor of Inductor and CapacitoradimeghaNo ratings yet
- Comparison and Contrast Essay FormatDocument5 pagesComparison and Contrast Essay Formattxmvblaeg100% (2)
- Installing Touareg R5 CamshaftDocument1 pageInstalling Touareg R5 CamshaftSarunas JurciukonisNo ratings yet
- Answers To Your Questions About Circumcision and HIV/AIDSDocument2 pagesAnswers To Your Questions About Circumcision and HIV/AIDSAlex BrownNo ratings yet
- Science 9 Q4 SML17 V2Document15 pagesScience 9 Q4 SML17 V2HotdogNo ratings yet
- University of Puerto Rico at PonceDocument16 pagesUniversity of Puerto Rico at Ponceapi-583167359No ratings yet
- Birding The Gulf Stream: Inside This IssueDocument5 pagesBirding The Gulf Stream: Inside This IssueChoctawhatchee Audubon SocietyNo ratings yet
- Mil STD 792fDocument13 pagesMil STD 792fdoradoanNo ratings yet
- ATI Respiratory PowerpointDocument90 pagesATI Respiratory PowerpointAnn KelseaNo ratings yet
- Join Our Telegram Channel: @AJITLULLA: To Get Daily Question Papers & SolutionsDocument24 pagesJoin Our Telegram Channel: @AJITLULLA: To Get Daily Question Papers & SolutionsNaveen KumarNo ratings yet
- Epicor Software India Private Limited: Brief Details of Your Form-16 Are As UnderDocument9 pagesEpicor Software India Private Limited: Brief Details of Your Form-16 Are As UndersudhadkNo ratings yet
- 2 Dawn150Document109 pages2 Dawn150kirubelNo ratings yet
- DX340LC: Crawler ExcavatorDocument20 pagesDX340LC: Crawler ExcavatorFeristha Meriani TabitaNo ratings yet