Professional Documents
Culture Documents
Crystallization Scaleup
Uploaded by
amoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Crystallization Scaleup
Uploaded by
amoCopyright:
Available Formats
Engineering Design and Scale
up of Crystallization
Professor A. B. Pandit
Institute of Chemical Technology
University of Mumbai
abp@udct.org
International Symposium on Crystallization, Filtration and Drying
MUICT, February 2006
2
Crystallization processes
Temperature
S
o
l
i
d
C
o
n
c
e
n
t
r
a
t
i
o
n
Dissolved Solid
Cooling
Crystallization
Crystallized
Amount
3
Crystallizer Operation
Lower Pressure
Solution fed at high
Temperature and pressure
Slurry
Vaporized solvent
Solubility exceeded
4
Classification of crystallizers
Classification based on the mode of operation
1. Batch crystallizer
2. Continuous crystallizer
Classification based on supersaturation
1. Supersaturation by cooling alone
a. Batch
b. Continuous
2. Supersaturation by adiabatic evaporation and cooling
a. Vacuum crystallizer
3. Supersaturation by evaporation
5
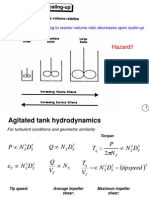
Agitated Tank crystallizer
Consist of cylindrical tank
provided with a low speed
agitator and cooling coil
Agitator improves heat
transfer, keeps the temperature
of the solution uniform and
keeps fine crystals in
suspension, which is essential
for their uniform growth.
Supersaturation is generated
by cooling
A known quantity of solution
is charged to the crystallizer.
6
By passing coolant through coil the mass in the crystallizer
cools due to heat transfer and the crystals are formed due to
decrease of solubility of solute in solvent
The mass is cooled to pre-decided temperature and finally
product stream containing crystals plus liquor is withdrawn for
the bottom of crystallizer
Main advantages are simple in construction and low initial cost
Main drawbacks of this crystallizer is that the deposited solids
on coil add resistance to heat transfer and efficiency of
crystallizer decreases
This type is used to produce fine chemicals, pharmaceutical
products and dye intermediates
7
Swenson-Walker crystallizer
It is a cooling type crystallizer
It consist of long open rectangular trough with semi cylindrical
bottom
Trough is jacketed externally for circulating cooling water during
operation
Spiral agitator rotating about 7 rpm is incorporated in the trough in
such a way that it is as close to the bottom of trough as possible
8
At one end of the crystallizer, inlet for hot solution is provided
and at other end of the crystallizer overflow gate for crystals and
mother liquor is provided
The hot, concentrated solution is fed at one end of the open
trough and flows slowly towards the other end of the trough
Water is fed to the jacket in such a way that if flows in counter
current fashion with respect to the solution flow
Spiral agitator keeps the crystals in suspension so that previously
formed crystals grows instead of formation of new crystals
The two-phase mixture of crystals and liquor leaves the
crystallizer through overflow gate
9
Oslo crystallizer
It is widely used when large
quantities of crystals of
controlled size are to be
produced
It consist of crystallizing
chamber, circulating pump and
external cooler for cooling the
solution
Feed solution to be crystallized
is fed from the top
Mother liquor from crystallizing chamber is withdrawn near feed
A with the help of circulating pump
It is then admitted the cooler E wherein supersaturation is
achieved by cooling
10
Supersaturated solution from cooler is fed back to the bottom of
crystallizing chamber through central pipe P that fluidizes the mass
in the tank
The mass during fluidization gets segregated according to particle
size
Larger particle are at bottom and finer particle at the top, get
recycled by pump through the heat exchanger
Below the feed point, there is supersaturated solution and the
particle grows further will go lower depending upon the circulation
velocity
Once crystals grow to required size, they are removed as a product
from the bottom of crystallizing chamber
11
Vacuum Crystallizer
Here supersaturation is
achieved by adiabatic
evaporative cooling
can be operated in both
mode i.e. batch and
continuous
used for the production of
large crystals
Continuous crystallizer
consist of tall vertical
cylindrical vessel with the
conical bottom, circulation
pump and vertical tubular
heat exchanger
12
Tangential inlet is provided on cylindrical vessel for introducing
hot solution
the magma from bottom of the vessel goes to the pump and it is
pumped through the vertical tubular heater and finally it enters
tangentially to the vessel
Flash evaporation of the solution takes place and produces
rapid cooling, resulting into supersaturation
suspension of crystals fed to centrifuge machine, crystals are
taken out as a product and mother liquor is recycled to down-
pipe
13
Fundamental Issues
Solid suspension and agitation mechanism
design
Near the walls solid particles can accumulate
Vaporization of solvent/Cooling/Heat Transfer
mechanism leads to gas generation
Particle Size Distribution
14
Single Particle Motion
Gravitational
Force
Buoyancy
Force
Drag
Force
g d
6
F
s
3
P G
t
=
g d
6
F
L
3
P B
t
=
2 2
P D D
V
2
1
d
4
C F
L
t
=
Taking a balance of all forces: Terminal velocity: Net force = 0
( )
2
t
2
P D L s
3
P
V
2
1
d
4
C g d
6
L
t
=
t
( )
L
D
L s P
t
C
g d
3
4
V
=
15
Terminal Settling Velocity
( )
L
L
D
s P
t
C
g d
3
4
V
=
Creeping Flow Regime Turbulent Flow Regime
1
V d
Re
L
L
t P
P
<<
=
P
D
Re
24
C =
( )
L
L
g d
18
1
V
s
2
P
t
=
200000
V d
Re 1000
L
L
t P
P
<
= <
44 . 0 C
D
=
( )
L
L
g d
75 . 1 V
s P
t
=
16
Typical Example
d
P
= 100 microns = 100 x 10
-6
m
L
= 1000 kg/m
3
,
S
= 1500 kg/m
3
Assume laminar flow:
( )
s / m 0027 . 0
18
g d
V
L
L
s
2
P
t
=
=
27 . 0
V d
Re
L
L t P
P
=
=
17
Impeller Classification
D/T Ratio
Shear / Flow
Open
Close
Radial
Axial
High
Shear
High
Flow
Flow Direction
18
Types of Impellers
Open Impellers: Low D/T ratio, high liquid
velocities in bulk of liquid
Disc turbine, pitched turbine
19
Types of Impellers
Close Clearance: High D/T ratio, High
velocities near the wall
Gate, Anchor, Helical Ribbon
20
Types of Impellers
Radial Flow: Generate primarily radially
outward flow
21
Types of Impellers
Axial Flow: Generate primarily axial flow
22
Important Parameters
Power Consumption
P = N
P
L
N
3
D
5
Flow Produced
Q = N
Q
N D
3
Power Dissipated per Unit mass (W/kg, kW/m
3
)
= P/M = N
P
L
N
3
D
5
/ (V
L
L
)
23
N
P
Re relationship is specific to impeller and
tank geometry
N
P
1/Re (Re < 10)
N
P
= f(Re) (Re > 10000)
Power Number
N
P
Re
24
Power Consumption
Installed Power
Power Drawn = 3 V I cos
If I = 30 Amps
Power Drawn = 1.732 x 415 x 30 x 0.85 = 18 kW
Electricity Consumption = 18 x 8000 kW hr /yr
Annual Cost = 18 x 8000 x 4 = Rs. 5.76 Lakhs/yr
25
Blending
Important Parameter is the Mixing Time
Time
C
o
n
c
e
n
t
r
a
t
i
o
n
Mixing Time
26
Typical mixing time speed relationship
N
mix
= constant = f (impeller, tank geometry)
Design Aspects for Blending
M
i
x
i
n
g
T
i
m
e
m
i
x
Impeller Speed N
27
Design Aspects for Blending
mix
time required for circulation
mix
V
L
/Q
mix
V
L
/N
Q
ND
3
Substituting for N using power per unit mass
mix
N
P
1/3
/ (N
Q
1/3
)
For a given power input, the energy efficiency
depends on power number as well as pumping
number
28
Typical Example
D = 1.6m, N
Q
= 1, N
P
= 2, N = 64rpm = 1.07rps
Q = 1 x 1.07 x 1.6
3
= 4.4 m
3
/s
Liquid volume = 30m
3
Mixing time = 5 x 30 / 4.4 = 34 s
Residence time = Crystallizer volume / Flowrate
of water = 30 / 85 = 0.35 hrs = 1270s
Power Consumed = N
P
L
N
3
D
5
= 1.4 x 1000 x 1.07
3
x1.6
5
= 18 kW
Power Consumed per unit volume = 18/30
= 0.6 kW/m
3
29
Solid Concentration Profiles
Radial Flow Impeller
30
Solid Concentration Profiles
Axial Flow Impeller
31
Design Aspects for Suspension
P
o
w
e
r
R
e
q
u
i
r
e
d
f
o
r
J
u
s
t
S
u
s
p
e
n
s
i
o
n
Particle Diameter or Particle Loading or settling Velocity
Down Flow Impeller
Radial Flow Impeller
Up Flow Impeller
32
Typical Example
30% Solids, d
P
=100m,
S
=1500 kg/m
3
T = 3.16m (30m
3
), D = 1.6 m, N
P
= 1.4
Terminal Settling Velocity = 0.003 m/s
Critical Impeller Speed for Suspension
N
CS
=1.15x(0.003)
0.28
x(30)
0.1
x(3.16/1.6)
0.8
/(1.6
0.85
)
=0.37 rps = 22 rpm (operating 64 rpm = 1.07 rps)
P
CS
= 1.4x1000x(0.37
3
)x(1.6
5
) = 0.743 kW
CS
= 0.743/30 = 0.03 kW/m
3
( = 0.6kW/m
3
)
) (T/D
D
X V
1.15 =
N
0.8
0.85
0.1 0.28
S
CS
33
Bubble column/Fluidized bed reactors
Similar analysis is required in terms of the
energy distribution patterns in the reactor
Efficiency of mixing and solid suspension is
strongly dependent on the quantum of the total
energy dissipated into the system as well as on
the fact that how the supplied energy is
distributed
34
Fundamental Issues
Solid suspension and agitator design
Near the walls solid particles can accumulate
Vaporization of solvent leads to gas generation
Particle Size Distribution
35
Prediction of Flow Patterns
Motion of fluids are governed by laws of
conservation of mass and momentum
Solving these equations allows us to predict
velocity fields within process equipment
These equations are partial differential equations
A set of such partial differential equations need to
be solved simultaneously
36
Model Formulation
Balance Equation:
In Out = Depleted Generated
Equation for:
(i) Total Mass
(ii) Momentum in 3 Directions
(iii) Turbulence Quantities
37
Predicted Velocity Fields
Case 1
Single PBTD
V
wall
= 1.4m/s
Case 3
Two Hydrofoils
V
wall
= 1.9m/s
Case 2
Gas foil PBTU
V
wall
= 0.6m/s
38
Prevention of Wall Build-up
Double Helical Ribbon Impeller
Gap b/w impeller tip and tank = 10 mm
Impeller Diameter = 3140 mm
Blade width = 310 mm
Pitch = 2200
Speed = 24 rpm, Power = 21 kW
Tip Speed (Wall Velocity) = 3.94 m/s
39
Fundamental Issues
Solid suspension and agitator design
Near the walls solid particles can accumulate
Vaporization of solvent leads to gas generation
Particle Size Distribution
40
Mass and enthalpy balance
Precipitation of solute
Iterative solution for T, F
L
and F
V
Solvent Vaporization in crystallizer
T
T, P, F
in
T, P, F
V
T, P, F
L
P
41
Predictions for a typical case
0.0
10.0
20.0
30.0
40.0
150 175 200 225 250 275 300
Temperature (deg C)
TA (wt%)
42
Fundamental Issues
Solid suspension and agitator design
Near the walls solid particles can accumulate
Vaporization of solvent leads to gas generation
Particle Size Distribution
43
Particle Size Distribution
(A Typical Case)
Mesh
size range
(micron)
Average size
(microns)
% by
weight
Cumulative
weight g
250 250-300 275 0.7 0.7
160 160-250 205 12.8 13.5
125 125-160 142.5 16.5 30.0
100 100-125 112.5 16.6 46.6
80 80-100 90 14.4 61.0
63 63-80 71.5 11.4 72.4
56 56-63 59.5 4.3 76.7
less than 56 0-56 28 23.3 100.0
44
PSD by Weight Fraction
PSD by Cumulative Weight
0
10
20
30
0 50 100 150 200 250 300
Average Size
wt%
0
25
50
75
100
0 20 40 60 80 100 120 140 160 180 200
Average Size
wt%
45
Crystallization Fundamentals
N is no. of crystals in a particular size range L
n = N/ L is the slope of above graph, number of
crystals per unit size
1.E+08
1.E+09
1.E+10
1.E+11
1.E+12
1.E+13
1.E+14
0 50 100 150 200 250 300
Average Size
N
o
.
o
f
p
a
r
t
i
c
l
e
s
i
n
g
i
v
e
n
s
i
z
e
r
a
n
g
e
46
Balance for Single crystallizer
V: volume
n: no. of crystals per unit size
G: growth rate
Q, C
i
Q, C
0
Population Balance for time interval t
and size range L = L
2
L
1
:
Input from feed = 0
Input by growth of Lower = n
1
G
1
V t
Output by growth = n
2
G
2
V t
Output by flow = Q n L t
At steady State, Input = output
|
.
|
\
|
t
=
t
= =
G
L
exp n n
g Integratin
G
n
) Q / V ( G
n
dL
dn
0
47
Laboratory scale experiments for determination
of Kinetic parameters
n = n
o
exp (-L/Gt)
Where n is the number of
nuclei at any time t of crystal
size L, n
o
is the embryo size
having diameter practically
equal to zero; G is the linear
growth rate and t is the mean
residence time of the crystals
n
L
48
Log n
L
n
o
Slope = 1/Gt and hence
Growth rate can be calculated
Y intercept gives the number of
nuclei that will be associated
by output
Rate of change of number of nuclei as a function of time
= dn
o
/dt = K
1
C
m
= n
o
G
G = dL/dt = K
2
C
n
49
Rearranging the earlier equations
1
2
m n
m
o
m
n
K
n G
K
| |
|
=
|
\ .
Laboratory experiments are required for the determination
of the constants in the above equation and hence the
growth rate and nucleation rate kinetic parameters
50
Application of mass and population balance
Predominant Crystal size gives the size of crystal consisting
largest mass fraction (L
D
)
Q
0
C
0
W
T
, n
Q
i
C
i
Solute mass balance
Q
i
C
i
= Q
0
(C
0
+ W
T
)
Now
W
T
= 6 K
V
n
0
(Gt)
4
Choose a mass fraction in a size range dL then
dW = K
V
nL
3
dL
( ) L W
dt
dW
=
- mass fraction in size range dL out of total mass present
dt
dW
51
( )
( )
4
0
3
6 t
G n K
dL nL K
L W
V
V
=
( )
( )
4
0
3
6 t G n
nL
dL
L W
=
i.e. the interval over which we are measuring the mass fraction
is dL
now ( )
( ) ( )
4
3
4
0
3
0
6
exp
6
exp
t
t
t
t
G
L
G
L
G n
L
G
L
n
dL
L W
|
.
|
\
|
=
|
.
|
\
|
=
( )
( ) ( )
2
4 4
3
3
6
exp
6
exp
1
L
G
G
L
G
L
G
L
G
dL
L W
dL
d
t
t
t
t t
|
.
|
\
|
+
|
.
|
\
|
|
.
|
\
|
=
|
.
|
\
|
now find the maxima
52
( )
( )
|
.
|
\
|
|
.
|
\
|
=
|
.
|
\
|
t t t
t
G
L
G
L
G
L
L
dL
L W
dL
d
G exp exp 3 6
3
2 4
|
.
|
\
|
|
.
|
\
|
=
t t t G
L
G
L
G
L
L
D D D
D
exp exp 3 0
3
2
(
(
|
.
|
\
|
=
t t G
L
L
G
L
D
D
D
3
2
3 exp 0
At maxima L = L
D
0 exp = |
.
|
\
|
t G
L
D
0 3
3
2
=
(
(
t G
L
L
D
D
or
t G
L
L
D
D
3
2
3 =
t G L
D
3 =
maximum size of crystal MSMPR = 3 x linear growth x Residence time
53
Design of batch crystallizer
In a batch crystallization, the weight and the number si changing
simultaneously
The number size distribution will not change but mass size
distribution will change with time
All the quantities will have finite number
Assume that No spontaneous nucleation occurs
i.e.
Hypothetically, we assume that the degree of supersaturation is
such that rate of nucleation is zero
0 =
dt
dN
T
54
Now Consider,
dL n L
dt
d
dt
dL
T
.. .
0
}
=
dL
dt
dL
n
dt
dn
L
dt
dL
T
}
+ =
0
.
}
=
0
nGdL
dt
dL
T
}
=
0
ndL G
dt
dL
T
T
T
GN
dt
dL
=
dt
dL
T
55
Similarly,
dL L n K
dt
d
dt
dA
a
T
..
2
0
}
=
dL L n
dt
d
K
dt
dA
a
T
) (
2
0
}
=
dL
dt
dL
L n
dt
dn
L K
dt
dA
a
T
}
+ =
0
2
. 2 .
GLdL n K
dt
dA
a
T
}
=
0
2
LdL n G K
dt
dA
a
T
}
=
0
2
T a
T
GL K
dt
dA
2 =
56
Also
dL K L n
dt
d
dt
dW
V
T
3
0
}
=
dL L n
dt
d
K
dt
dW
V
T
) (
3
0
}
=
dL
dt
dL
L n
dt
dn
L K
dt
dW
V
T
}
+ =
0
2 3
3
}
=
0
2
3 nGdL L K
dt
dW
V
T
}
=
0
2
3 ndL L G K
dt
dW
V
T
a
T
V
T
K
A
G K
dt
dW
3 =
57
In the case of batch crystallization, there is control over rate of
evaporation or rte of cooling
If we know rate of precipitation, we can control rate of
evaporation or rate of condensation so that we get crystals of
specific size
58
Ideal classified Bed Crystallizer
Assumptions
1. No uncontrolled nucleation occurs
2. A constant input of seed crystals per unit time is assumed. Thus these
seed crystals will come out as product
Number of crystals in Crystallizer at any time = t
3. The size of the seed crystals is specified as L
0
and is assumed to be the
smallest size crystal in the Crystallizer
4. The crystal bed is perfectly classified and can be subdivided in to layers
comprising of crystals of equal sizes having equal extension time
Seed crystals will occupy top layer. They will grow, settling velocity
increases and they move to lower layer. This continues till they reach
bottom layer with size L
P
. They must have spent equal time in each layer to
reach this size.
59
( )
n
G
C A K
dt
dW
A =
It should be noted that growth rate is always in linear dimension
whereas the Growth kinetics is not always linear.
6. The crystal shape can be represented by geometrical body
characterized by single dimension L and area & volume shape
factor.
A = K
a
L
2
V = K
V
L
3
7. Steady state operation
5. The crystal growth kinetics can be explained by
60
Under steady state condition
Difference in degree of supersaturation = amount of growth experienced
by crystals
Balance for the bottom layer having product
Q (AC
P
- AC) = K
V
(L
p
3
L
3
) -------- (1)
AC
P
- degree of supersaturation of entering liquid
AC degree of supersaturation of liquid leaving last layer
K
V
(L
p
3
L
3
) volume change
K
V
(L
p
3
L
3
) mass change
K
V
(L
p
3
L
3
) total mass change
Crystal production rate
P = K
V
L
p
3
(kg/hr) ------ (2)
From eq. (1) and (2)
61
|
|
.
|
\
|
A = A
3
3
1
P
P
L
L
Q
P
C C
From this, we can find rate of change of supersaturation required in
each layer to increase crystal size from L to L
P
A
T
= K
a
L
2
N
T
N
T
total number of crystals in Crystallizer i.e. N
T
= t
Hence A
T
= K
a
L
2
N ------- (4)
This is per unit volume i.e unit volume of Crystallizer contains N
crystals
W = K
V
L
3
N --------- (5)
dW = 3 K
V
NL
2
dL ---------- (6)
Substitute this in crystals growth kinetics
62
( )
n
g
V
a
C K
K
K
dt
dL
G A = =
3
n
P
P g
V
a
L
L
Q
P
C K
K
K
dt
dL
G
(
(
|
|
.
|
\
|
A = =
3
3
1
3
|
|
.
|
\
|
A = A
3
3
0
0
1
P
P
L
L
Q
P
C C
( ) A = A 1
0 P
C C
Since L
0
>> L
P
Overall balance
P P
C
C
C Q
P
A
A
=
A
=
0
1
where
63
n
P
P
L
L
G G
(
(
|
|
.
|
\
|
=
3
3
1 1
( )
n
P g
V
A
P
C K
K
K
G A =
3
Where
G
P
is maximum possible linear growth rate because AC
P
is
maximum supersaturation
If = 1, it is called total de-saturation
n
P
P
L
L
G G
dt
dL
3
|
|
.
|
\
|
= =
There is going to be difference between residence time of crystal and
residence time of fluid
} }
=
dt G dL L L
P
L
L
n n
P
P
O
3 3
i.e.
64
t
P
L
L
n
n
p
G
n
L
L
P
=
(
0
3 1
3 1
3
( )
( ) t =
n n
P
P
n
P
L L
n G
L
3 1
0
3 1
3
1 3
( )
t =
|
|
.
|
\
|
1 3 1 3
0
3
1 1
1 3
n
P
n
P
n
P
L L
n G
L
( )
t =
|
|
.
|
\
|
1 3 1 3
0
1 3
0
1 3 3
1 3
n
P
n
n n
P
P
n
P
L L
L L
n G
L
( )
t =
(
(
|
|
.
|
\
|
1
1 3
1 3n
O
P
P
P
L
L
n G
L
65
let n= 1
t =
(
(
|
|
.
|
\
|
1
2
1 3n
O
P
P
P
L
L
G
L
if we know the ratio of L
P
to L
O
, we can calculate t for fluid
if = 0, G = G
P
P
P
G
L L
0
= t
Overall balance for crystallization
=
P
L
L
i i V
L n K W
0
3
3
3
P
L
L
W
t
~
- Crystal production rate
66
4
t P
W =
Now
}
=
P
L
L
P
dL L
L L
0
3
0
3
1
t
( )
4
1
4
0
4
0
3
L L
L L
P
P
= t
( )( )
4
0
2
0
2
3
L L L L
P P
+ +
= t
4
3
3
P
L
= t
P
L 63 . 0 = t
If L
P
>>L
0
67
4 unit time per weight crystal
weight crystal t
=
4
crystal by spent time
t
=
if the product crystal growth time is 4 times the mean
residence time i.e overall crystal residence time, this means that
allowing the process of crystallization, the crystal goes through
circulation loop 4 times before it comes out as product crystal
Thus liquid residence time and crystal residence time is same
But product crystal residence is 4 times the overall crystal
residence time
Thus before we take product out the product must have spent
time equal to 4 times the residence time of fluid.
|
|
.
|
\
|
P
P
G
L L
0
i.e.
68
Assuming nucleation only in 1st crystallizer
and constant growth rate in all crystallizers
PSD is measured at the end of 5th crystallizer
n
5
values are calculated from this data
n
o
and G can be fitted to match the plant data
These values can be used for prediction
What is the effect of Residence Time on PSD?
Crystallizers in Series
4
0 5
G
L
exp 1
G
L
exp n n
(
|
.
|
\
|
t
|
.
|
\
|
t
=
69
PSD Predictions
0
25
50
75
100
0 20 40 60 80 100 120 140 160
Average Size (micron)
% wt.
30% Decrease in
Residence Time
10% Decrease in
Residence Time
MPS=106
MPS=75
70
Parameters for scale-up of crystallization
1. Identical solid and liquid flow characteristics
2. Identical supersaturation in all zones of the crystallization
3. Identical seed crystal characteristics
4. Identical magma densities
Since crystal settling characteristics depend on magma densities,
to maintain identical flow characteristics, requires identical flow
characteristics
5. Identical contact time
It is very difficult to maintain all these conditions in a continuous
stirred tank crystallizers, however can maintain these conditions
in a draft tube crystallizer. Hence scale up of draft tube is easier
71
Criteria for Scale up
Constant Reynolds Number and Froude number
Reynolds Number = Liquid Density Stirrer speed (Diameter )
2
Viscosity
Froude Number = Diameter (Stirrer speed)
2
Gravitational acceleration
Achieving constant values is however extremely difficult if not
impossible
72
Concluding Remarks
Selection of equipment type for the specific
application under question is crucial
Determination of parameters for nucleation
rate and growth rate kinetics using laboratory
scale experiments
Well defined scale up criteria will be the key
to success of efficient operation of the
industrial crystallizer
You might also like
- Closed Feed Water HeatersDocument56 pagesClosed Feed Water HeatersAnudeep ChittluriNo ratings yet
- Crystallization Technology HandbookDocument782 pagesCrystallization Technology HandbookkhadidjaBOUCHENTOUF100% (3)
- Tonnage CalculationDocument3 pagesTonnage CalculationEmba MadrasNo ratings yet
- Large Chilled Water SystemDocument314 pagesLarge Chilled Water SystemEsteban Lopez Arboleda100% (3)
- Fundamentals of INdustrial ControlDocument5 pagesFundamentals of INdustrial ControlKirtikumarNo ratings yet
- 1962 Fallout Shelter DesignDocument218 pages1962 Fallout Shelter DesignLouie_popwhatski100% (1)
- CrystallizationDocument27 pagesCrystallizationaaaNo ratings yet
- Crystallization Equipment PDFDocument37 pagesCrystallization Equipment PDFMirza Salkić100% (1)
- ReactorsDocument7 pagesReactorsLyka BalmesNo ratings yet
- Crystal IzationDocument10 pagesCrystal IzationJavier Eduardo Penagos VazquezNo ratings yet
- CrystallizationDocument29 pagesCrystallizationYawar QureshiNo ratings yet
- Centrifugal FiltrationDocument43 pagesCentrifugal FiltrationDaniel Andre Ocampo Prudencio100% (1)
- Swenson CrystallizationDocument12 pagesSwenson Crystallization陳盈佃No ratings yet
- Evaporator SDocument23 pagesEvaporator SMehwish NoorNo ratings yet
- Continuous CrystallizersDocument22 pagesContinuous CrystallizersAravind Vicky0% (1)
- Fluid Mixing Senior ReportDocument21 pagesFluid Mixing Senior ReportAnonymous 3aOOjQNo ratings yet
- Cooling Tower DriveDocument32 pagesCooling Tower DriveHassan KhanNo ratings yet
- Cooling Tower Capacity CalculationDocument2 pagesCooling Tower Capacity CalculationPurusotaman BarthibanNo ratings yet
- Agitated Nutsche Filter: Chemicals, Food Products, Pharmaceuticals, Drug Intermediates IndustriesDocument2 pagesAgitated Nutsche Filter: Chemicals, Food Products, Pharmaceuticals, Drug Intermediates IndustrieskiranNo ratings yet
- Chiller Plant DesignDocument48 pagesChiller Plant Designryxor-mrbl100% (1)
- ExtractionDocument10 pagesExtractionetayhailuNo ratings yet
- NABET Scheme Version 3 INFORMATIONDocument60 pagesNABET Scheme Version 3 INFORMATIONamo0% (1)
- FiltrationDocument77 pagesFiltrationmeet2abhayNo ratings yet
- CrystallizationDocument43 pagesCrystallizationKathleen May Dayao0% (1)
- Crystallizer DesignDocument20 pagesCrystallizer DesignJabel Pates100% (11)
- Chemical Reactor Analysis and Applications for the Practicing EngineerFrom EverandChemical Reactor Analysis and Applications for the Practicing EngineerNo ratings yet
- Lectuer-11 EvaporatorDocument41 pagesLectuer-11 EvaporatorAurenio RibeiroNo ratings yet
- CrystallizationDocument30 pagesCrystallizationMuhamadYazid50% (4)
- Design For EvaporatorDocument5 pagesDesign For EvaporatorDevang GodhaniyaNo ratings yet
- Bubble Column ReactorsDocument34 pagesBubble Column ReactorsGhaya Bani Rushaid100% (2)
- Chapter-Cooling TowersDocument17 pagesChapter-Cooling TowersSAGIS ETIENNENo ratings yet
- Bubble Cap Plate For Distillation ColumnDocument26 pagesBubble Cap Plate For Distillation Columnsanjukec100% (2)
- Extraction MethodsDocument3 pagesExtraction MethodsMuhammadRafiqNo ratings yet
- List of Least Learned Competencies With Interventions ConductedDocument2 pagesList of Least Learned Competencies With Interventions ConductedGerry Chel-Nunez Awa-Laurente Aga100% (1)
- EIA ReportDocument183 pagesEIA ReportamoNo ratings yet
- Mto I Class 9 10Document128 pagesMto I Class 9 10Mriganabh SarmaNo ratings yet
- RFL-C1500-C6000 Continuous Wave FibeLaser User GuideDocument39 pagesRFL-C1500-C6000 Continuous Wave FibeLaser User Guidevijayrockz06100% (1)
- Crystallizer SelectionDocument8 pagesCrystallizer SelectionKTINE08No ratings yet
- Mixing and AgitationDocument17 pagesMixing and AgitationTa Den April88% (8)
- Heat and Mass TransferDocument23 pagesHeat and Mass TransferMaria Cecille Sarmiento GarciaNo ratings yet
- Advantages of Adhesive in DentistryDocument29 pagesAdvantages of Adhesive in DentistryAnonymous CY62A9No ratings yet
- Construction and Demolition WasteDocument81 pagesConstruction and Demolition WasteamoNo ratings yet
- CrystallizerDocument10 pagesCrystallizerNAGARAJAN A R [CB.EN.U4CHE17035]No ratings yet
- Crystallization EquipmentDocument37 pagesCrystallization EquipmentMadeline Ngo100% (10)
- Packed Bed FermentersDocument20 pagesPacked Bed FermentersGerald Owen FranzaNo ratings yet
- CG5052 - Agitation and Mixing - 2 - 2015 PDFDocument32 pagesCG5052 - Agitation and Mixing - 2 - 2015 PDFzuopengxiangNo ratings yet
- Batch Reactor: Optical High Precision Components by Hellma in The European Columbus Space LaboratoryDocument16 pagesBatch Reactor: Optical High Precision Components by Hellma in The European Columbus Space Laboratoryangelie_gubatanNo ratings yet
- CentrifugationDocument53 pagesCentrifugationseeyo123No ratings yet
- Rachel Adams Jana Dengler Megan Macleod Kyla Sask Rachel Adams Jana Dengler Megan Macleod Kyla SaskDocument31 pagesRachel Adams Jana Dengler Megan Macleod Kyla Sask Rachel Adams Jana Dengler Megan Macleod Kyla Saskbetengaan2100% (1)
- CRYSTALLIZER DESIGN (Rev02)Document33 pagesCRYSTALLIZER DESIGN (Rev02)Shai Sta CatalinaNo ratings yet
- Chapter#8 CrystallizationDocument49 pagesChapter#8 Crystallization07216738950% (1)
- 6 Crystallizer Design and Operation1Document22 pages6 Crystallizer Design and Operation1Shweta ChaudhariNo ratings yet
- Multiple Effect EvaporationDocument4 pagesMultiple Effect EvaporationDianne VillanuevaNo ratings yet
- CrystallizerDocument2 pagesCrystallizernas_101303No ratings yet
- 9 EvaporationDocument82 pages9 Evaporationsanjay YadavNo ratings yet
- BT 0413 BioseparationTechnologyLaboratoryDocument21 pagesBT 0413 BioseparationTechnologyLaboratoryvijaygovindarajNo ratings yet
- Agitation and Mixing (My Presentation)Document22 pagesAgitation and Mixing (My Presentation)Aleem Choudhry100% (1)
- MSG CrystallizerDocument22 pagesMSG CrystallizerRonel MendozaNo ratings yet
- Enteric Coated Aspirin Tablets FinalDocument72 pagesEnteric Coated Aspirin Tablets Finalronak_panchal_21No ratings yet
- The Production of Acetylsalicylic AcidDocument45 pagesThe Production of Acetylsalicylic AcidAhmed Ali100% (1)
- Packed Bed AbsorptionDocument4 pagesPacked Bed AbsorptionSenthilNathanNo ratings yet
- Chapter 3 Separation Processes (Unit Operations)Document10 pagesChapter 3 Separation Processes (Unit Operations)almutaz9879No ratings yet
- Sterilization 2Document8 pagesSterilization 2Rhia100% (1)
- Chapter 4 Reactor DesignDocument16 pagesChapter 4 Reactor DesignAli AhsanNo ratings yet
- Class 1 Size Reduction ZZDocument43 pagesClass 1 Size Reduction ZZZen EbeNo ratings yet
- Heat Transfer Lab Manual 2015-16Document99 pagesHeat Transfer Lab Manual 2015-16Harshit Sinha100% (1)
- Evaporator: CH250 - Heat TransferDocument21 pagesEvaporator: CH250 - Heat TransferManas AkashNo ratings yet
- Separation Processes Lab ReportDocument15 pagesSeparation Processes Lab ReportArslanQureshi0% (1)
- CRE-II - Chapter-04 Fluid-Particle Systems - HKB 2.10.18Document42 pagesCRE-II - Chapter-04 Fluid-Particle Systems - HKB 2.10.18Ananya DaveNo ratings yet
- Film Evaporation TechnologyDocument12 pagesFilm Evaporation TechnologyAditya Bayu SalaksaNo ratings yet
- Project Report 2 - Final - Design 1 AliffDocument142 pagesProject Report 2 - Final - Design 1 AliffAdi PutraNo ratings yet
- Fluidized Bed ReactorDocument23 pagesFluidized Bed ReactorHelberth Lopes50% (2)
- Epa Water Treatment Manual Primary Secondary Tertiary1Document131 pagesEpa Water Treatment Manual Primary Secondary Tertiary1Pamela RichardsonNo ratings yet
- Solvents Economic Study ReportDocument129 pagesSolvents Economic Study Reportamo100% (1)
- Epa Water Treatment Manual Primary Secondary Tertiary1Document131 pagesEpa Water Treatment Manual Primary Secondary Tertiary1Pamela RichardsonNo ratings yet
- Epa Water Treatment Manual Primary Secondary Tertiary1Document131 pagesEpa Water Treatment Manual Primary Secondary Tertiary1Pamela RichardsonNo ratings yet
- Rain Water Harvesting CPWD MANUAL PDFDocument84 pagesRain Water Harvesting CPWD MANUAL PDFRv SinghNo ratings yet
- Safety AwarenessDocument11 pagesSafety AwarenessamoNo ratings yet
- Tata Report Aug8Document66 pagesTata Report Aug8amoNo ratings yet
- Bombay Minor Mineral Ext Rules-1955Document37 pagesBombay Minor Mineral Ext Rules-1955amo100% (1)
- MOEF Guideline For Env LabDocument122 pagesMOEF Guideline For Env LabamoNo ratings yet
- Cus Tic Software EnglishDocument43 pagesCus Tic Software EnglishamoNo ratings yet
- Dispersion Air Pollution ModellingDocument44 pagesDispersion Air Pollution ModellingamoNo ratings yet
- General Standards Industry Effluents Emissions Pollution - CPCB IndiaDocument16 pagesGeneral Standards Industry Effluents Emissions Pollution - CPCB IndiaVaishnavi JayakumarNo ratings yet
- Aloha TechDocument96 pagesAloha Techrufino.perea.2No ratings yet
- Hazardous Wastes Management Handling and Transboundary Movement Rules 2008Document40 pagesHazardous Wastes Management Handling and Transboundary Movement Rules 2008anpuselvi125No ratings yet
- IDRISI Software ManualDocument32 pagesIDRISI Software Manualamo100% (1)
- Nabet Version 3Document156 pagesNabet Version 3amoNo ratings yet
- EIA EMP Report of JSW SteelDocument879 pagesEIA EMP Report of JSW Steelamo75% (4)
- Coal Treatment For Ash ContentDocument6 pagesCoal Treatment For Ash ContentamoNo ratings yet
- OSHA 2236 - Materials Handling and StorageDocument41 pagesOSHA 2236 - Materials Handling and StorageWahed Mn ElnasNo ratings yet
- Boiler CalculationsDocument19 pagesBoiler CalculationsamoNo ratings yet
- AAQM GuidelinesDocument164 pagesAAQM GuidelinesKrishna KumarNo ratings yet
- Eia Process Flow Chart: ContDocument2 pagesEia Process Flow Chart: Contshreek16No ratings yet
- Sustainable Cities: Canadian Reality or Urban Myth?Document10 pagesSustainable Cities: Canadian Reality or Urban Myth?amoNo ratings yet
- New NABET SchemeDocument168 pagesNew NABET SchemeamoNo ratings yet
- New Standard TOR - 5 (F)Document8 pagesNew Standard TOR - 5 (F)amoNo ratings yet
- Stoichiometry of Chemical ReactionDocument4 pagesStoichiometry of Chemical ReactionKristian KuliNo ratings yet
- Project - Silicon Solar Cell-CoDocument55 pagesProject - Silicon Solar Cell-CoSudheer SebastianNo ratings yet
- Conduction A Long Simple BarDocument5 pagesConduction A Long Simple Bardiaa ibrahimNo ratings yet
- B SafeunitDocument4 pagesB SafeunitSabariyantoNo ratings yet
- Moisture Content in PET PreformsDocument3 pagesMoisture Content in PET PreformsGeorge MarkasNo ratings yet
- L 19 - Mse628a - 30 10 19Document25 pagesL 19 - Mse628a - 30 10 19Dhanishtha SinghNo ratings yet
- Business PlanDocument2 pagesBusiness PlanSakunthalaPanditharatneNo ratings yet
- MathsDocument2 pagesMathsAditya Singh PatelNo ratings yet
- Class Xi Physics Annual Exam 2017 18Document3 pagesClass Xi Physics Annual Exam 2017 18Anupam TiwariNo ratings yet
- Chapter 2 Modern Control SystemsDocument19 pagesChapter 2 Modern Control SystemsDilamo FelekeNo ratings yet
- H Beam Weight-TWC PDFDocument1 pageH Beam Weight-TWC PDFSankar CdmNo ratings yet
- Segui 6e ISM Ch08Document105 pagesSegui 6e ISM Ch08miraj patelNo ratings yet
- Lawal Habeeb Babatunde: A Seminar Report OnDocument21 pagesLawal Habeeb Babatunde: A Seminar Report OnAyinde AbiodunNo ratings yet
- A New Static Induction Thyristor (Sith) Analytical Model: Jue Wang and Barry W. WilliamsDocument11 pagesA New Static Induction Thyristor (Sith) Analytical Model: Jue Wang and Barry W. WilliamsAbdulAzizNo ratings yet
- Earth and Life Science Copy (Repaired)Document39 pagesEarth and Life Science Copy (Repaired)Aaron Manuel MunarNo ratings yet
- RAO, Capitulo 14, VibracionesDocument61 pagesRAO, Capitulo 14, Vibracioneskazekage2009No ratings yet
- Matlab Fourier Series Signal & SystemDocument15 pagesMatlab Fourier Series Signal & SystemNik Ahmad FaisalNo ratings yet
- Om Asu12r2 FRDocument19 pagesOm Asu12r2 FRmacdrewNo ratings yet
- Materiales CompuestosDocument167 pagesMateriales CompuestosTatiana MerchanNo ratings yet
- Identification of Transport Mechanism in Adsorbent Micropores From Column DynamicsDocument9 pagesIdentification of Transport Mechanism in Adsorbent Micropores From Column DynamicsFernando AmoresNo ratings yet
- PWM DC Motor ControllerDocument11 pagesPWM DC Motor ControllerSanthosh Kumar100% (1)
- Nonlinear Systems: Lyapunov Stability Theory - Part 2Document36 pagesNonlinear Systems: Lyapunov Stability Theory - Part 2giacomoNo ratings yet
- Theory AssignmentDocument12 pagesTheory Assignmentfahadfadi48No ratings yet