Professional Documents
Culture Documents
Isentropic Efficiencies of Turbines, Compressors and Nozzles
Uploaded by
grandecaciqueOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Isentropic Efficiencies of Turbines, Compressors and Nozzles
Uploaded by
grandecaciqueCopyright:
Available Formats
Isentropic Efficiencies of Turbines,
Compressors and Nozzles
Lecture # 28
Nov. 3, 08
Announcements
Midterm-II: Friday, Nov. 7
th
, 11 am, in class
10 problems from Chapters 4,5,6
4 from Ch. 4, 2 from Ch.5, 4 from Ch. 6
Todays material is NOT included. Entropy Balances for control
volumes (HW # 10) is included.
Total Points: 20
Review Session in Class on Wednesday
Homework # 10 Solutions will be posted on Wed. morning
Equation sheet will contain all Key Equations listed at end of
chapters 1-6; Cover page with unit conversions will be
provided. Where necessary property tables will be given
Isentropic Processes
A process for which the entropy is constant, is called
an isentropic process. On the h-s diagram and on the
T-s diagram, we can draw the processes, as shown in
the next figures. The state at the end of the isentropic
process can be obtained by matching the initial
entropy and another property such as pressure or
specific volume or temperature.
Fig06_08
F
i
g
0
6
_
0
9
Ideal Gas Model
Let us now derive some analytical relations for an
isentropic process for an ideal gas. We can write
]
) ( ) (
exp[
) ln( ) ( ) (
) ln( ) ( ) (
R
T s T s
p p
p
p
R T s T s
p
p
R T s T s
1
0
2
0
1 2
1
2
1
0
2
0
1
2
1
0
2
0
0
=
+ =
=
Constant Specific Heats
Let us consider an isentropic process for an ideal gas
when the specific heats are constants.
Introducing the ideal gas relations,
|
|
.
|
\
|
+
|
|
.
|
\
|
=
|
|
.
|
\
|
|
|
.
|
\
|
=
1
2
1
2
1
2
1
2
0
0
v
v
R
T
T
c
p
p
R
T
T
c
v
P
ln ln
ln ln
1 1
=
=
k
R
c
k
kR
c
v P
;
Constant Specific Heats
We can then derive the following relations from the
above equations:
Substituting the above relation by eliminating the
temperature ratio from the above relation, we can
write:
The process is shown in the following figure
) ( / ) (
) ( ) (
1
2
1
1
2
1
1
2
1
2
= =
k k k
v
v
T
T
p
p
T
T
constant ; ) ( = =
k k
pv or
v
v
p
p
2
1
1
2
Fig06_10
Isentropic Efficiencies
Isentropic efficiencies involve a comparison between
the actual performance of a device and the
performance that would be achieved under idealized
circumstances for the same inlet state and the same
exit pressure.
Isentropic Turbine Efficiency
For a turbine with no heat transfer and KE and PE
effects neglected, we can write an expression for rate
of work as:
2 1
h h
m
W
CV
=
Fig06_E6
Isentropic Efficiency, Turbine
The maximum value for rate work is obtained for the
smallest value of permitted h
2
for the given exit
pressure. This value is obtained using the second law
constraint. Since there is no heat transfer,
The smallest value of s
2
corresponds to a reversible
process, with s
2
= s
1
. All sates to the left of the
isentropic vertical lines are not possible. The
corresponding enthalpy for isentropic process is h
2s.
.
0
1 2
> = s s
m
CV
o
Turbine Isentropic Efficiency
The turbine isentropic efficiency is defined as:
Typical values of isentropic efficiencies are between
0.7 and 0.9.
s
s
CV
CV
t
h h
h h
m
W
m
W
2 1
2 1
=
|
|
.
|
\
|
=
q
Isentropic Efficiency of Compressors
For compressors, we want to the minimum amount of
work. The actual work is given by:
The minimum work corresponds to an isentropic
process (since there is no heat transfer). Hence
); (
1 2
h h
m
W
CV
=
); ( ) (
1 2
h h
m
W
s s
CV
=
F
i
g
0
6
_
1
2
Isentropic Efficiency of Compressors
The isentropic efficiency of a compressor is defined as:
Typical values for isentropic efficiency of a compressor
is between 75 and 85%.
Isentropic pump efficiency can be defined similarly.
1 2
1 2
h h
h h
m W
m W
s
CV
s CV
C
=
) / (
) / (
q
Nozzles
Since nozzles produce kinetic energy from a drop in
enthalpy, the nozzle isentropic efficiency is defined as:
For well-designed nozzles, the isentropic efficiencies
are around 95%, hence nozzles are fairly easy to
design free of internal irreversibilities.
2
2
2
2
2
2
/
/
s
n
V
V
= q
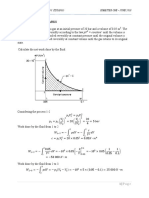
Sample Problem 2
A steam turbine operates at steady state with inlet
conditions of p
1
= 5 bar, T
1
= 320
o
C. Steam leaves the
turbine at a pressure of 1 bar. There is no significant
heat transfer between the turbine and surroundings.
KE and PE effects are negligible. If the isentropic
efficiency is 75%, determine the work developed per
unit mass flowing through the turbine, in kJ/kg.
Given: Turbine inlet and outlet conditions, isentropic
efficiency. KE and PE effects are negligible, and no
heat transfer.
Fig06_E6
Solution
The work developed can be calculated from the
isentropic efficiency and the isentropic work. Evaluate
the following properties:
h
1
= 3105.6 kJ/kg. s
1
= 7.5308 kJ/kg.K The exit
pressure is 1 bar, and if for an isentropic process, the
exit entropy is 7.5308 kJ/kg.K. The corresponding h
2s
=
2743.0 kJ/kg. Substituting the values for the inlet and
isentropic enthalpy, the actual work is:
kg kJ
m
W
CV
/ . ) . . ( . 95 271 0 2743 6 3105 75 0 = =
Sample Problem 2
Steam enters a nozzle operating at steady state at p
1
=
140 lbf/in
2
and T
1
= 600
o
F with a velocity of 100 ft/s.
The pressure and temperature at the exit are p
2
= 40
lbf/in
2
and T
2
= 350
o
F. There is no significant heat
transfer between nozzle and its surroundings and
changes in PE between inlet and exit can be
neglected. Determine the nozzle isentropic efficiency.
Solution: The nozzle efficiency is defined as the ratio
of actual Kinetic energy developed to the isentropic
value
Fig06_E6
Solution
For the actual operation,
The inlet enthalpy for T
1
= 600
o
F and p
1
= 140lbf/in
2
is
1326.4 Btu/lbm; s
1
= 1.7191 Btu/lbm.
o
R
T
2
= 350
o
F, p
2
= 40 lbf/in
2
, h
2
= 1211.8 Btu/lb. Hence
) (
2 1
2
1
2
2
2 2
h h
V V
+ =
lbm Btu
lbm Btu lbm Btu
V
/ .
/ ) . . ( /
) )( . )( (
) (
8 114
8 1211 4 1326
778 2 32 2
100
2
2 2
2
=
+ =
Solution
Now we need to evaluate h
2s
for the isentropic
process. Match pressure = 40 lbf/in
2
, and s
2
= s
1
=
1.7191 Btu/lbm.
o
R results in h
2s
= 1202.3 Btu/lbm
%) . ( .
.
.
)
/ .
/ ) . . ( /
) )( . )( (
) (
)
4 92 924 0
3 124
8 114
2
2
3 124
8 1202 4 1326
778 2 32 2
100
2
2
2
2
2
2 2
2
= = =
=
+ =
s
s
V
V
efficiency Isentropic
lbm Btu
lbm Btu lbm Btu
V
You might also like
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationFrom EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationNo ratings yet
- Turbines and CompressorsDocument70 pagesTurbines and CompressorsAkshay Deshpande100% (1)
- Thermofluids & Engine: Gas Power CyclesDocument29 pagesThermofluids & Engine: Gas Power CyclesQim SvNo ratings yet
- Turbo-Machinery (Meng3201) : Chapter-3Document28 pagesTurbo-Machinery (Meng3201) : Chapter-3Asnake Bahiru100% (1)
- LAT4Document6 pagesLAT4Said FerdjallahNo ratings yet
- Gas Engine FundamentalsDocument13 pagesGas Engine Fundamentalsherdin56No ratings yet
- Crude Preheat Train With Fired HeaterDocument3 pagesCrude Preheat Train With Fired HeaterhaosfNo ratings yet
- Applied Thermodynamics Exam 2018 Wirh SolutionsDocument9 pagesApplied Thermodynamics Exam 2018 Wirh SolutionsFarouk BassaNo ratings yet
- Turbo Machines Lab: Centrifugal and Reciprocating CompressorsDocument38 pagesTurbo Machines Lab: Centrifugal and Reciprocating CompressorsUpendra SravanNo ratings yet
- Assessment of CompresorsDocument14 pagesAssessment of CompresorsranveerNo ratings yet
- Pressure Ake Int Absolute Stage Last of Pressure e Arg Disch AbsoluteDocument8 pagesPressure Ake Int Absolute Stage Last of Pressure e Arg Disch Absolutemasih tadayonNo ratings yet
- Turbines - Types-Wps OfficeDocument5 pagesTurbines - Types-Wps OfficerkNo ratings yet
- Rotary Screw CompressorDocument16 pagesRotary Screw CompressorIlija LončarNo ratings yet
- Turbomachinery Selection Exploitation and MaintenanceDocument9 pagesTurbomachinery Selection Exploitation and MaintenanceMichael Chikwendu100% (1)
- Transportasi Fluida (Compressible Fluid-Fan Blower)Document36 pagesTransportasi Fluida (Compressible Fluid-Fan Blower)Evia Salma ZauridaNo ratings yet
- Reciprocating Compressor PDFDocument28 pagesReciprocating Compressor PDFmoNo ratings yet
- BlowersDocument58 pagesBlowersmahmad61100% (1)
- Centrifugal Compressor NotesDocument23 pagesCentrifugal Compressor NotesSubhash Padmanabhan100% (1)
- Gas Compression IIDocument13 pagesGas Compression IIAnuraag MulpuriNo ratings yet
- Session 5 - Linde Hampson Process.Document7 pagesSession 5 - Linde Hampson Process.SHOBHIT KUMARNo ratings yet
- Lect 5 - Liquefaction - 2015 PDFDocument6 pagesLect 5 - Liquefaction - 2015 PDFAnonymous oqlnO8e100% (1)
- Centrifugal Compressors For CPI Plants PDFDocument4 pagesCentrifugal Compressors For CPI Plants PDFAmanda Aracely Herreria SalazarNo ratings yet
- Compressors - Written ReportDocument17 pagesCompressors - Written ReportJenina Rosa P. LlanesNo ratings yet
- Gas Turbine in Cairo North Power StationDocument38 pagesGas Turbine in Cairo North Power StationAbdul Moeed Kalson0% (1)
- Engineering Lesson Guide 11: Gas Turbine TheoryDocument24 pagesEngineering Lesson Guide 11: Gas Turbine TheoryIwan RuhiyanaNo ratings yet
- At AssignmentDocument3 pagesAt AssignmentYogesh PatilNo ratings yet
- Distillation Column Reboiler Selection, Sizing and TROUBLESHOOTING, Kolmetz Handbook of Process Equipment DesignDocument13 pagesDistillation Column Reboiler Selection, Sizing and TROUBLESHOOTING, Kolmetz Handbook of Process Equipment DesignMAGESHKUMAR GNo ratings yet
- Process Equipment Design-1Document15 pagesProcess Equipment Design-1Nikunj Thummar100% (1)
- Unit-7 - Steam and Gas TurbineDocument24 pagesUnit-7 - Steam and Gas TurbineKedir Mohammed100% (1)
- Reciprocating & Rotary CompressorDocument64 pagesReciprocating & Rotary Compressorraghu.entrepreneur100% (1)
- C Module-5 Reciprocating Air Compressors. Reciprocating Compressors - Construction - WorkingDocument48 pagesC Module-5 Reciprocating Air Compressors. Reciprocating Compressors - Construction - WorkingJasraj Gill100% (1)
- Screw CompressorDocument23 pagesScrew CompressorvaibhavNo ratings yet
- Assignment 1Document4 pagesAssignment 1Ziyad Awali100% (1)
- Gas Compressor: 1.types of CompressorsDocument9 pagesGas Compressor: 1.types of CompressorsSimona Gheorghe100% (1)
- Rotary Screw ProcessDocument9 pagesRotary Screw ProcessEagle WingsNo ratings yet
- APPLIED THERMODYNAMICS 18ME42 Module 02 Question No 3a-3b & 4a-4bDocument26 pagesAPPLIED THERMODYNAMICS 18ME42 Module 02 Question No 3a-3b & 4a-4bThanmay JSNo ratings yet
- Subsonic Ejector RamjetDocument99 pagesSubsonic Ejector Ramjetmarco20874No ratings yet
- TurbomachinesDocument2 pagesTurbomachinesThesinghNo ratings yet
- Gas CompressorDocument6 pagesGas Compressorrandhawa435No ratings yet
- Sizing Forced Draft Fans For Fired HeatersDocument5 pagesSizing Forced Draft Fans For Fired Heatersdlalameen4471No ratings yet
- Reciprocating Air CompressorsDocument20 pagesReciprocating Air CompressorsAshleen MarshallNo ratings yet
- Centrifugal CompressorDocument14 pagesCentrifugal CompressorridanormaNo ratings yet
- Api 611 5Th Edition General " Purpose Steam Turbine Existing TurbineDocument1 pageApi 611 5Th Edition General " Purpose Steam Turbine Existing TurbineAlejandro GilNo ratings yet
- Heat Exchangers ReportDocument16 pagesHeat Exchangers Report刘羿村No ratings yet
- The Axial Flow Compressor CompromiseDocument8 pagesThe Axial Flow Compressor Compromisekincandia100% (4)
- Or YorkDocument32 pagesOr YorkJavier BarbeytoNo ratings yet
- Process Flow Diags Study - Ver2Document9 pagesProcess Flow Diags Study - Ver2Sergey KorenevskiyNo ratings yet
- Centrifugal AxialcompressorsDocument28 pagesCentrifugal Axialcompressorsultrasonic81100% (1)
- Positive Displacement CompressorsDocument46 pagesPositive Displacement CompressorsMahendra PuguhNo ratings yet
- Pur-15-02 - Air Purger Type PurDocument4 pagesPur-15-02 - Air Purger Type PurAnderson Giovanny Herrera DelgadoNo ratings yet
- Gas Turbine - PDFDocument214 pagesGas Turbine - PDFSreepriodas RoyNo ratings yet
- Centrifugal Compressors: Fabrizio Tani October 23rd 2001Document41 pagesCentrifugal Compressors: Fabrizio Tani October 23rd 2001Stefano CaidominiciNo ratings yet
- Isentropic Process PDFDocument2 pagesIsentropic Process PDFJeebee Logroño AloNo ratings yet
- Domestic Refrigerator AnalysisDocument4 pagesDomestic Refrigerator AnalysisHoracio RodriguezNo ratings yet
- 125452033-Thermodynamics D.J.Dunn PDFDocument185 pages125452033-Thermodynamics D.J.Dunn PDFkalite123No ratings yet
- Problem Set G 10.213 Solution Spring 2002 23) A Schematic of The Rankine Cycle and Representation On A T-S Diagram: Q 2 TDocument10 pagesProblem Set G 10.213 Solution Spring 2002 23) A Schematic of The Rankine Cycle and Representation On A T-S Diagram: Q 2 TRalph Segundo SalvadorNo ratings yet
- Reviewlecture-I 20081001 48e3c2399f4d65 74115154Document37 pagesReviewlecture-I 20081001 48e3c2399f4d65 74115154Austin BarrilleauxNo ratings yet
- THER206 Tut 2nd LawDocument2 pagesTHER206 Tut 2nd Law5432167890OOOONo ratings yet
- Chapter Five PDFDocument24 pagesChapter Five PDFعبدالله رعد حران 32No ratings yet
- Despiece Gamma 2 3HP 100 150 Lts 2Document2 pagesDespiece Gamma 2 3HP 100 150 Lts 2grandecaciqueNo ratings yet
- Weld Nugget Development and Integrity in Resistance Spot Welding of High-Strength Cold-Rolled Sheet SteelsDocument8 pagesWeld Nugget Development and Integrity in Resistance Spot Welding of High-Strength Cold-Rolled Sheet SteelsgrandecaciqueNo ratings yet
- Hydrogen-Induced Cracking Susceptibility in High-Strength Weld MetalDocument6 pagesHydrogen-Induced Cracking Susceptibility in High-Strength Weld MetalgrandecaciqueNo ratings yet
- Welding of A Normalized High Strength Low Alloy Plate Steel of Structural QualityDocument8 pagesWelding of A Normalized High Strength Low Alloy Plate Steel of Structural QualitygrandecaciqueNo ratings yet
- Weld Nugget Development and Integrity in Resistance Spot Welding of High-Strength Cold-Rolled Sheet SteelsDocument8 pagesWeld Nugget Development and Integrity in Resistance Spot Welding of High-Strength Cold-Rolled Sheet SteelsgrandecaciqueNo ratings yet
- Welding of A Normalized High Strength Low Alloy Plate Steel of Structural QualityDocument8 pagesWelding of A Normalized High Strength Low Alloy Plate Steel of Structural QualitygrandecaciqueNo ratings yet
- Industrial Production of BioinsecticidesDocument11 pagesIndustrial Production of BioinsecticidesNwigwe Promise ChukwuebukaNo ratings yet
- Competencies - Gen Chem 1Document5 pagesCompetencies - Gen Chem 1Chi StephNo ratings yet
- MPA4112Document128 pagesMPA4112José Esteban Mascareña VázquezNo ratings yet
- Science ParadoxDocument6 pagesScience ParadoxMahendra JayanNo ratings yet
- Energy StoredDocument6 pagesEnergy StoredMae chelle San AndresNo ratings yet
- Ka TableDocument2 pagesKa TableMuhammad AimanNo ratings yet
- Enzyme Controlled Reaction LabDocument2 pagesEnzyme Controlled Reaction Labapi-291218692No ratings yet
- The Geographers Tools PDFDocument27 pagesThe Geographers Tools PDFAhou Ania Qouma JejaNo ratings yet
- In-Situ Spectroscopic Studies of Adsorption at The Electrode and ElectrocatalysisDocument10 pagesIn-Situ Spectroscopic Studies of Adsorption at The Electrode and ElectrocatalysisEudes SantosNo ratings yet
- Answer Scheme BIOLOGY Paper 3 PRA 2007Document6 pagesAnswer Scheme BIOLOGY Paper 3 PRA 2007Ferguson TehNo ratings yet
- 2-Vle Part 2Document22 pages2-Vle Part 2Arfa Zulkifli01No ratings yet
- CHE-504 Lecture 4 Basics of Mass Spectrometery by Dr. Charu C. PantDocument13 pagesCHE-504 Lecture 4 Basics of Mass Spectrometery by Dr. Charu C. PantAbhishek Singh ChandelNo ratings yet
- CH 10Document34 pagesCH 10hirenpatel_universalNo ratings yet
- Quantum Reality May Be Incomplete, Einstein Argument ShowsDocument4 pagesQuantum Reality May Be Incomplete, Einstein Argument ShowsAndy HoNo ratings yet
- Chemistry: Classification of MatterDocument29 pagesChemistry: Classification of MatterRamzen Raphael DomingoNo ratings yet
- Franz Diffusion Cell Approach For Pre-Formulation Characterisation of Ketoprofen Semi-Solid Dosage FormsDocument8 pagesFranz Diffusion Cell Approach For Pre-Formulation Characterisation of Ketoprofen Semi-Solid Dosage FormsHEMANo ratings yet
- Acidity, Alkalinity, and HardnessDocument30 pagesAcidity, Alkalinity, and HardnessCharan DeepNo ratings yet
- Chapter 1-Thermodynamic-Merged-CompressedDocument60 pagesChapter 1-Thermodynamic-Merged-CompressedAina SyafiqahNo ratings yet
- Chemical Reactor Design-CHEM-E7135: Yongdan LiDocument57 pagesChemical Reactor Design-CHEM-E7135: Yongdan Likiranpatil1014532No ratings yet
- Physics of Cosmic AccelerationDocument37 pagesPhysics of Cosmic Accelerationnow_priyankaNo ratings yet
- 1983 Book Atomistics of FractureDocument1,043 pages1983 Book Atomistics of FractureHuynh ThuongNo ratings yet
- Deccan TrapsDocument5 pagesDeccan TrapsNTA UGC-NETNo ratings yet
- B Bogdanov 2Document6 pagesB Bogdanov 2tonmoyahmed06No ratings yet
- Vernier Calipers (Procedure) - Class 11 - Physics - Amrita Online LabDocument3 pagesVernier Calipers (Procedure) - Class 11 - Physics - Amrita Online Labgetashishvaid100% (1)
- CHE 202 TUTORIAL QUESTIONSDocument6 pagesCHE 202 TUTORIAL QUESTIONSFawziyyah AgboolaNo ratings yet
- Jet Impingement Cooling in Gas Turbines For Improving Thermal Efficiency and Power DensityDocument21 pagesJet Impingement Cooling in Gas Turbines For Improving Thermal Efficiency and Power DensityDileep ElangovanNo ratings yet
- PHY 310 Modern Physics Course OutlineDocument6 pagesPHY 310 Modern Physics Course OutlineNur HamizahNo ratings yet
- Dual Nature of Radiation: in 1 ShotDocument64 pagesDual Nature of Radiation: in 1 ShotDisney DoreamonNo ratings yet
- Water Resources: Edward D. SchroederDocument31 pagesWater Resources: Edward D. SchroederAdrian LozadaNo ratings yet
- PHIL University Physics For The Physical and Life Sciences Volume 2 PDFDocument694 pagesPHIL University Physics For The Physical and Life Sciences Volume 2 PDFws1751367% (3)