Professional Documents
Culture Documents
Radio Pharmaceutics
Uploaded by
DrGaurav TiwariOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Radio Pharmaceutics
Uploaded by
DrGaurav TiwariCopyright:
Available Formats
Radiopharmaceutics
What is Radiopharmacy?
Radiopharmacy = Nuclear Pharmacy
Nuclear pharmacy is a specialty area of
pharmacy practice dedicated to the
compounding and dispensing of
radioactive materials for use in nuclear
medicine procedures.
Introduction:
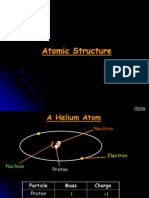
All substances are made of atoms.
These have electrons (e) around the outside
(negatively charged),
and a nucleus in the middle.
The nucleus consists of protons (positively charged) and
neutrons (neutral).
The atomic number of an atom is the number of
protons in its nucleus.
The atomic mass is the number of protons + neutrons
in its nucleus.
Introduction:
Isotopes of an atom have the same number of protons, but a
different number of neutrons.
Example:
Consider a carbon atom:
It has 6 protons and 6 neutrons - we call it "carbon-12" because
it has an atomic mass of 12 (6 plus 6).
One useful isotope of carbon is "carbon-14", which has 6 protons
and 8 neutrons.
Radioisotopes, Radionuclides: unstable isotopes which are
distinguishable by radioactive transformation.
Radioactivity: the process in which an unstable isotope undergoes
changes until a stable state is reached and in the transformation
emits energy in the form of radiation (alpha particles, beta particles
and gamma rays).
Introduction:
Radiation refers to particles or waves coming from the
nucleus of the atom (radioisotope or radionuclide) through
which the atom attempts to attain a more stable
configuration.
Types of radioactivity:
How to produce a radioactive nuclide ?
1- Natural radioactivity:
Nuclear reactions occur spontaneously
2- Artificial radioactivity:
The property of radioactivity produced by particle
bombardment or electromagnetic irradiation.
A- Charged-particle reactions
e.g. protons (
1
1
H)
e.g. deuterons (
2
1
H)
e.g. alpha particles (
4
He)
Types of radioactivity:
B- Photon-induced reactions
The source of electromagnetic energy may be gamma-
emitting radionuclide or high-voltage x-ray generator.
C- Neutron-induced reactions
- It is the most widely used method
- It is the bombardment of a nonradioactive target nucleus
with a source of thermal neutrons.
Production of radionuclides:
1- Charged particle bombardment
Radionuclides may be produced by bombarding target
materials with charged particles in particle accelarators
such as cyclotrons.
- A cyclotron consists of :
Two flat hollow objects called dees.
The dees are part of an electrical circuit.
On the other side of the dees are large magnets that (drive)
steer the injected charged particles (protons, deutrons,
alpha and helium) in a circular path
The charged particle follows a circular path until the particle
has sufficient energy that it passes out of the field and
interact with the target nucleus.
Cyclotron
Production of radionuclides:
2- Neutron bombardment
Radionuclides may be produced by bombarding target
materials with neutrons in nuclear reactors
- The majority of radiopharmaceuticals are produced by
this process
Production of radionuclides: :
3- Radionuclide generator systems
Principle:
A long-lived parent radionuclide is allowed to decay to its
short-lived daughter radionuclide and the latter is
chemically separated in a physiological solution.
Example:
- technetium-99m, obtained from a generator constructed
of molybdenum-99 absorbed to an alumina column.
Eluted from the column with normal saline
99Mo/99mTc Generator:
Parent: 99Mo as molybdate
Half-life: 66 hr.
Decays by - emission, gamma: 740, 780 keV.
High affinity to alumina compared to .
Daughter: as pertechnetate
Adsorbent Material: Alumina (aluminum oxide, )
Eluent: saline (0.9% NaCl)
Eluate:
Radioactive decay:
The rate of decay can be described by:
N = N
o
e
-t
where N is the number of atoms at elapsed time t, N
o
is the
number of atoms when t = 0, and is the disintegration
constant characteristic of each individual radionuclide.
T = 0.693 /
The intensity of radiation can be described by:
I = I
0
e - 0.693/ T
1/2
Radioactive Decay Law
Radioactive decay:
Half life symbol t
1/2
the time taken for the activity of
a given amount of a radioactive substance to decay to
half of its initial value.
Total activity symbol A number of decays an object
undergoes per second.
Radionuclidic purity- is that percentage of the total
radioactivity that is present in the form of the stated
radionuclide.
Mode of radioactive decay:
Radioactive decay is the process in which an unstable
atomic nucleus spontaneously loses energy by emitting
ionizing particles and radiation.
This decay, or loss of energy, results in an atom of one
type, called the parent nuclide transforming to an atom of
a different type, named the daughter nuclide.
When an unstable nucleus decays, It may give out:-
1- Alpha particle decay:
Alpha particles are made of 2 protons and 2
neutrons.
We can write them as , or , because
they're the same as a helium nucleus.
This means that when a nucleus emits an alpha
particle, its atomic number decreases by 2 and its
atomic mass decreases by 4.
Alpha particles are relatively slow and heavy.
They have a low penetrating power - you can
stop them with just a sheet of paper.
Because they have a large charge, alpha particles
ionise other atoms strongly.
Alpha-decay occurs in very heavy elements, for
example, Uranium and Radium.
Since alpha particles cannot penetrate the dead layer of the skin, they do
not present a hazard from exposure external to the body.
However, due to the very large number of ionizations they produce in a
very short distance, alpha emitters can present a serious hazard when they
are in close proximity to cells and tissues such as the lung. Special
precautions are taken to ensure that alpha emitters are not inhaled,
ingested or injected.
2- Beta particle decay:
Beta particles have a charge of minus 1. This
means that beta particles are the same as an
electron.
We can write them as or , because
they're the same as an electron.
This means that when a nucleus emits a -
particle: the atomic mass is unchanged
the atomic number increases or
decreases by 1.
They are fast, and light.
Beta particles have a medium penetrating
power - they are stopped by a sheet of
aluminium.
Example of radiopharmaceutical emits ,
phosphorus-32
Beta particles ionise atoms that they pass, but not
as strongly as alpha particles do.
Beta particles are much less massive and less charged than
alpha particles and interact less intensely with atoms in the
materials they pass through, which gives them a longer range
than alpha particles.
3- Gamma ray:
Gamma rays are waves, not particles.
This means that they have no mass and no
charge.
in Gamma decay:
- atomic number unchanged
- atomic mass unchanged.
Gamma rays have a high penetrating power
- it takes a thick sheet of metal such as lead to
reduce them.
Gamma rays do not directly ionise other
atoms, although they may cause atoms to
emit other particles which will then cause
ionisation.
We don't find pure gamma sources - gamma
rays are emitted alongside alpha or beta
particles.
3- Gamma ray:
Useful gamma sources inculde Technetium-99m, which
is used as a "tracer" in medicine.
This is a combined beta and gamma source, and is
chosen because betas are less harmful to the patient
than alphas (less ionisation) and because Technetium
has a short half-life (just over 6 hours), so it decays away
quickly and reduces the dose to the patient.
Alpha particles are easy to stop,
gamma rays are hard to stop.
Mode of radioactive decay:
Type of Radiation Alpha particle Beta particle Gamma ray
Symbol
or
Charge +2 -1 0
Speed slow fast Very fast
Ionising ability high medium 0
Penetrating power low medium high
Stopped by: paper aluminium
lead
Radiation measurement:
( R) the roentgen for exposure:
Is the amount of radiation that produces ionization of one
electrostatic unit of either positive or negative charge per cubic
centimeter of air at 0 C and 760 mmHg.
(rad) radiation absorbed dose is a more universal unit, it is a measure of
the energy deposited in unit mass of any material by any type of
radiation.
(rem) has been developed to account for the differences in effectiveness
of different radiations in causing biological damage.
Rem = rad RBE
RBE is the relative biological effectiveness of the radiation.
Radiation measurement:
The basic unit for quantifying radioactivity (i.e. describes the
rate at which the nuclei decay).
Curie (Ci):
Curie (Ci), named for the famed scientist Marie Curie
Curie = 3.7 x 10
10
atoms disintegrate per second (dps)
Millicurie (mC
i
) = 3.7 x 10
7
dps
Microcurie (uC
i
) = 3.7 x 10
4
dps
Becquerel (Bq):
A unit of radioactivity. One becquerel is equal to 1
disintegration per second.
Properties of an Ideal Diagnostic
Radioisotope:
Types of Emission:
Pure Gamma Emitter: (Alpha & Beta Particles are
unimageable & Deliver High Radiation Dose.)
Energy of Gamma Rays:
Ideal: 100-250 keV e.g.
Suboptimal:<100 keV e.g.
>250 keV e.g.
Photon Abundance:
Should be high to minimize imaging time
Properties of an Ideal Diagnostic
Radioisotope:
Easy Availability:
Readily Available, Easily Produced & Inexpensive:
e.g.
Target to Non target Ratio:
It should be high to:
maximize the efficacy of diagnosis
minimize the radiation dose to the patient
Effective Half-life:
It should be short enough to minimize the radiation dose
to patients and long enough to perform the procedure.
Ideally 1.5 times the duration of the diagnostic
procedure.
Properties of an Ideal Diagnostic
Radioisotope:
Example: For a Bone Scan which is a 4-h procedure,
99mTc- phosphate compounds with an effective half-life of
6 h are the ideal radiopharmaceuticals
Patient Safety:
Should exhibit no toxicity to the patient.
Preparation and Quality Control:
Should be simple with little manipulation.
No complicated equipment
No time consuming steps
Preparation of Radiopharmaceutical
1- Sterilization:
- Radiopharmaceutical preparations intended for
parenteral administration are sterilized by a suitable
method.
- Terminal sterilization by autoclaving is recommended for
heat stable products
- For heat labile products, the filteration method is
recommended.
2- Addition of antimicrobial preservatives:
- Radiopharmaceutical injections are commonly supplied
in multidose containers.
Preparation of Radiopharmaceutical :
The requirement of the general monograph for
parenteral preparations that such injections should
contain a suitable antimicrobial preservative in a suitable
concentration does not necessarily apply to
radiopharmaceutical preparations.
A reason for this exemption is that many common
antimicrobial preservatives (for example, benzyl alcohol)
are gradually decomposed by the effect of radiation in
aqueous solutions.
3- Compounding:
compounding can be as simple as:
- adding a radioactive liquid to a commercially available
reagent kit
as complex as:
1- the creation of a multi-component reagent kit
N.B. Kit for radiopharmaceutical preparation
means a sterile and pyrogen-free reaction vial containing the
nonradioactive chemicals [e.g., complexing agent (ligand),
reducing agent, stabilizer, or dispersing agent] that are
required to produce a specific radiopharmaceutical after
reaction with a radioactive component.
2- the synthesis of a radiolabeled compound via a multi-step
preparation process.
3- Compounding:
The process of compounding radiopharmaceuticals must be
under the supervision of recognized nuclear physician or a
radiopharmacist.
STABILITY OF COMPOUNDED PREPARATIONS
All extemporaneously compounded parenteral
radiopharmaceutical preparations should be used no more
than 24 hours post compounding process unless data are
available to support longer storage.
Radiation shielding:
Adequate shielding must be used to protect
laboratory personnel from ionizing
radiation.
Pro-Tec II Syringe Shield
Guard Lock PET Syringe Shield
Color Coded Vial Shields
Pro-Tec V Syringe Shield
Vial Shield
Unit Dose Pig
High Density Lead Glass Vial
Shield
Sharps Container Shields
Radiation shielding:
Alpha and beta radiations are readily shielded because of
their limited range of penetration.
The alpha particles are mono-energetic and have a range
of a few centimetres in air.
aluminium, glass, or transparent plastic materials, are
used to shield sources of beta radiation.
Gamma radiation is commonly shielded with lead and
tungsten.
Radiopharmaceutical quality control:
Visual Inspection of Product
- Visual inspection of the compounded radiopharmaceutical
shall be conducted to ensure the absence of foreign
matter and also to establish product identity by confirming
that
(1) a liquid product is a solution, a colloid, or a suspension
(2) a solid product has defined properties that identify it.
Assessment of Radioactivity
-The amount of radioactivity in each compounded
radiopharmaceutical should be verified and documented
prior to dispensing, using a proper standardized
radionuclide (dose) calibrator.
Radiopharmaceutical quality control:
Radionuclidic Purity
- Radionuclidic purity can be determined with the use of a
suitable counting device
-The gamma-ray spectrum, should not be significantly
different from that of a standardized solution of the
radionuclide.
Radiochemical purity
- Radiochemical purity is assessed by a variety of
analytical techniques such as:
- liquid chromatography - paper chromatography
- thin-layer chromatography - electrophoresis
the distribution of radioactivity on the chromatogram is
determined.
Radiopharmaceutical quality control:
Verification of Macroaggregate Particle Size and
Number
pH
Microbiological Control (sterility test) and Bacterial
Endotoxin Testing
Radiopharmaceutical quality control:
Labelling
The label on the outer package should include:
a statement that the product is radioactive or the
international symbol for radioactivity
the name of the radiopharmaceutical preparation;
the preparation is for diagnostic or for therapeutic use;
the route of administration;
the total radioactivity present (for example, in MBq per
ml of the solution)
the expiry date
the batch (lot) number
for solutions, the total volume;
any special storage requirements with respect to
temperature and light;
the name and concentration of any added microbial
preservative
Application of radiopharmaceuticals:
1- Treatment of disease:
(therapeutic radiopharmaceuticals)
They are radiolabeled molecules designed to deliver
therapeutic doses of ionizing radiation to specific diseased
sites.
Chromic phosphate P32 for lung, ovarian, uterine, and
prostate cancers
Sodium iodide I 131 for thyroid cancer
Samarium Sm 153 for cancerous bone tissue
Sodium phosphate P 32 for cancerous bone tissue and
other types of cancers
Strontium chloride Sr 89 for cancerous bone tissue
2- As an aid in the diagnosis of disease (diagnostic
radiopharmaceuticals)
The radiopharmaceutical accumulated in an organ of interest emit
gamma radiation which are used for imaging of the organs with the
help of an external imaging device called gamma camera.
- Radiopharmaceuticals used in tracer techniques for measuring
physiological parameters (e.g.
51
Cr-EDTA for measuring glomerular
filtration rate).
- Radiopharmaceuticals for diagnostic imaging
(e.g.
99m
TC-methylene diphosphonate (MDP) used in bone scanning).
Application of radiopharmaceuticals:
You might also like
- Radiopharmaceutics: Nuclear Medicine's Essential ToolsDocument52 pagesRadiopharmaceutics: Nuclear Medicine's Essential ToolsalibinaminNo ratings yet
- Radiography Testing Study GuideDocument52 pagesRadiography Testing Study GuideManish SinghNo ratings yet
- IGCSE Physics Atomic Structure NotesDocument5 pagesIGCSE Physics Atomic Structure NotesAishath WaheedaNo ratings yet
- Physics (Radioactivity)Document21 pagesPhysics (Radioactivity)Hery HadzrenNo ratings yet
- RADIOACTIVITYDocument26 pagesRADIOACTIVITYHarIsh SangwanNo ratings yet
- 4.7 RadioactivityDocument14 pages4.7 Radioactivitygabrielsuva6No ratings yet
- Topic 5 Atomic Physics Notes PDFDocument5 pagesTopic 5 Atomic Physics Notes PDFpreeti.2405100% (1)
- Class 10 Physics Chapter 12 Revision NotesDocument4 pagesClass 10 Physics Chapter 12 Revision NotesGAMING IN MOODNo ratings yet
- The Atom: Particle ChargeDocument10 pagesThe Atom: Particle ChargedilsharakaviNo ratings yet
- 7 1 NotesDocument17 pages7 1 Notesapi-182809945No ratings yet
- Radiopharmaceuticals 1Document56 pagesRadiopharmaceuticals 1salinaNo ratings yet
- Chemistry Nuclear ActivityDocument5 pagesChemistry Nuclear Activityron espejoNo ratings yet
- Detecting & Measuring RadioactivityDocument7 pagesDetecting & Measuring RadioactivityChetan GandhiNo ratings yet
- Nuclear Tech English - PDFDocument20 pagesNuclear Tech English - PDFShivang BhardwajNo ratings yet
- Radioactivity and Nuclear Physics ExplainedDocument18 pagesRadioactivity and Nuclear Physics ExplainedJovicaSutevNo ratings yet
- Health AND Safety in Biomedical EngineeringDocument7 pagesHealth AND Safety in Biomedical EngineeringxutemNo ratings yet
- Act. 2 Natural Radioactive SeriesDocument2 pagesAct. 2 Natural Radioactive Seriesxiejie22590No ratings yet
- Detect Radioactivity and Atomic StructureDocument61 pagesDetect Radioactivity and Atomic Structureshruti shahNo ratings yet
- Radiation Safety: ἄτομος or átomos meaning "indivisible") is the smallest particle of aDocument10 pagesRadiation Safety: ἄτομος or átomos meaning "indivisible") is the smallest particle of adypietNo ratings yet
- Understanding Atomic StructureDocument9 pagesUnderstanding Atomic StructureNashae Hall-Pass AllenNo ratings yet
- Radio Activity and ParticlesDocument34 pagesRadio Activity and ParticlesKiron SheiqNo ratings yet
- Radiographic Testing Techniques and ApplicationsDocument99 pagesRadiographic Testing Techniques and ApplicationsThe Engineers EDGE, Coimbatore0% (1)
- Radiation Sources, Effects, and SolutionsDocument89 pagesRadiation Sources, Effects, and SolutionsCM NajitoNo ratings yet
- Radiation Properties GuideDocument17 pagesRadiation Properties GuideJagroopSinghBalhraNo ratings yet
- Background Radiation Notes in 38 CharactersDocument15 pagesBackground Radiation Notes in 38 Charactersanwar9602020No ratings yet
- Radioactivity Revision NotesDocument4 pagesRadioactivity Revision NotesJohnNo ratings yet
- Radiopharmaceutical NotesDocument6 pagesRadiopharmaceutical NotesARUN NTNo ratings yet
- Radiation Chemistry Notes EditedDocument15 pagesRadiation Chemistry Notes Editedkiama kariithiNo ratings yet
- Radiation FundamentalsDocument46 pagesRadiation FundamentalsRichard mwanaNo ratings yet
- Module 3 - Chem LecDocument5 pagesModule 3 - Chem LecErianne ReyesNo ratings yet
- Nuclear Chemistry and EnergyDocument18 pagesNuclear Chemistry and EnergyJm EscobarNo ratings yet
- 10.1 Understanding The Nucleus of An Atom 10.1.1 Composition of The NucleusDocument14 pages10.1 Understanding The Nucleus of An Atom 10.1.1 Composition of The NucleusrajhiniNo ratings yet
- 8.1 - Nuclear RadiationDocument9 pages8.1 - Nuclear RadiationasNo ratings yet
- RadioactivityDocument17 pagesRadioactivityNurasfiqah AKNo ratings yet
- Chapter 22 Nuclear Chem Study GuideDocument5 pagesChapter 22 Nuclear Chem Study GuideVicky100% (2)
- Folio Radioactivity Ting 5Document16 pagesFolio Radioactivity Ting 5akunaruto92100% (3)
- What Is An Atom?: Mass NumberDocument4 pagesWhat Is An Atom?: Mass NumberCampy BcNo ratings yet
- Radiopharmaceutics PDFDocument22 pagesRadiopharmaceutics PDFahmedesmail282005No ratings yet
- RadioactivityDocument16 pagesRadioactivityNur HafezaNo ratings yet
- Atoms Nucleus and Radiation - To Be Filled inDocument15 pagesAtoms Nucleus and Radiation - To Be Filled inNick RandazzoNo ratings yet
- Radioanalytical Techniques: Ali Abbas M.Phil Pharmaceutical ChemistryDocument54 pagesRadioanalytical Techniques: Ali Abbas M.Phil Pharmaceutical ChemistryAdia MasooraNo ratings yet
- Radioactivity: Alpha Decay: During Alpha Decay, The Nucleus of An Atom Emits An Alpha Particle, WhichDocument2 pagesRadioactivity: Alpha Decay: During Alpha Decay, The Nucleus of An Atom Emits An Alpha Particle, WhichEhtasham EhtashamNo ratings yet
- Terminologies: 1. Alpha ParticlesDocument7 pagesTerminologies: 1. Alpha ParticlesRalph MazinoNo ratings yet
- Beta and Gamma RaysDocument10 pagesBeta and Gamma RaysEnoch SpalbarNo ratings yet
- P2 Radiation and StarsDocument24 pagesP2 Radiation and StarsSteve Bishop100% (1)
- Greg Herman Environmental Health and Safety Radiation Protection 814-865-6391 Gsh12@psu - EduDocument132 pagesGreg Herman Environmental Health and Safety Radiation Protection 814-865-6391 Gsh12@psu - EduSpoiled BratNo ratings yet
- S.4. Modern Physics-1 - Read and PracticeDocument13 pagesS.4. Modern Physics-1 - Read and PracticeKEIFER SUTHERLANDNo ratings yet
- Basic Radiation PhysicsDocument31 pagesBasic Radiation PhysicseetuaNo ratings yet
- 3 Alpha Beta Gamma - tcm18-17765Document5 pages3 Alpha Beta Gamma - tcm18-17765VenkgNo ratings yet
- Chapter 3 Nuclear RadiationDocument164 pagesChapter 3 Nuclear RadiationJoseph MabajenNo ratings yet
- RadiationDocument23 pagesRadiationbananaboy29.macdonaldNo ratings yet
- Physics Form 5 Chapter 5Document22 pagesPhysics Form 5 Chapter 5Charlene87% (15)
- Radioactivity - AnswersDocument4 pagesRadioactivity - Answersapi-269764684No ratings yet
- AtomicDocument41 pagesAtomicHassanKMNo ratings yet
- Nuclear ChangeDocument59 pagesNuclear ChangeKo NwayNo ratings yet
- Chemistry Nuclear Chem NotesDocument18 pagesChemistry Nuclear Chem Notesmonishsarker564No ratings yet
- Everything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksFrom EverandEverything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksNo ratings yet
- PrescriptionDocument30 pagesPrescriptionDrGaurav TiwariNo ratings yet
- Article Wjpps 1386011514Document7 pagesArticle Wjpps 1386011514Donig FermanianNo ratings yet
- Bio-Compatible and Bio - Degradable PolymersDocument29 pagesBio-Compatible and Bio - Degradable PolymersDrGaurav TiwariNo ratings yet
- Approaches and Techniques in Performance AppraisalDocument5 pagesApproaches and Techniques in Performance AppraisalDrGaurav TiwariNo ratings yet
- Approaches and Techniques in Performance AppraisalDocument5 pagesApproaches and Techniques in Performance AppraisalDrGaurav TiwariNo ratings yet
- Drug Release Kinetics ModelsDocument7 pagesDrug Release Kinetics ModelsSajid Khan SadozaiNo ratings yet
- Important DocumentDocument2 pagesImportant DocumentDrGaurav TiwariNo ratings yet
- Approaches and Techniques in Performance AppraisalDocument5 pagesApproaches and Techniques in Performance AppraisalDrGaurav TiwariNo ratings yet
- Metronidazole-Loaded Bioabsorbable Films As Local Antibacterial Treatment of Infected Periodontal PocketsDocument8 pagesMetronidazole-Loaded Bioabsorbable Films As Local Antibacterial Treatment of Infected Periodontal PocketsDrGaurav TiwariNo ratings yet
- Mpharma PharmaceuticsDocument14 pagesMpharma PharmaceuticsDrGaurav TiwariNo ratings yet
- Approaches and Techniques in Performance AppraisalDocument5 pagesApproaches and Techniques in Performance AppraisalDrGaurav TiwariNo ratings yet
- Uppsc Fee CollectionDocument2 pagesUppsc Fee CollectionDrGaurav TiwariNo ratings yet
- 2009 Drug InformationDocument4 pages2009 Drug InformationXee JayNo ratings yet
- Pharmaceutical Sales Training Excellence: Tools, Processes & Resources That Drive EffectivenessDocument17 pagesPharmaceutical Sales Training Excellence: Tools, Processes & Resources That Drive EffectivenessDrGaurav TiwariNo ratings yet
- Radio PharmaceuticsDocument52 pagesRadio PharmaceuticsDrGaurav TiwariNo ratings yet
- QaDocument3 pagesQaDrGaurav TiwariNo ratings yet
- Management of IPR in IndiaDocument12 pagesManagement of IPR in IndiaDrGaurav TiwariNo ratings yet
- Management of IPR in IndiaDocument12 pagesManagement of IPR in IndiaDrGaurav TiwariNo ratings yet
- Application Form-PGTD TEACHERS 05 Feb 2013Document8 pagesApplication Form-PGTD TEACHERS 05 Feb 2013DrGaurav TiwariNo ratings yet
- Automation in Pharmaceutical ManufacturingDocument6 pagesAutomation in Pharmaceutical ManufacturingDrGaurav TiwariNo ratings yet
- 1st Middle East Six Sigma ForumDocument29 pages1st Middle East Six Sigma ForumanandweNo ratings yet
- CopyrightDocument27 pagesCopyrightDrGaurav Tiwari0% (1)
- Bhavya TQMDocument34 pagesBhavya TQMDrGaurav TiwariNo ratings yet
- Container and ClosureDocument9 pagesContainer and ClosureDrGaurav TiwariNo ratings yet
- ImplantsDocument2 pagesImplantsDrGaurav TiwariNo ratings yet
- Application Form-PGTD TEACHERS 05 Feb 2013Document8 pagesApplication Form-PGTD TEACHERS 05 Feb 2013DrGaurav TiwariNo ratings yet