Professional Documents
Culture Documents
Inactivated Whole-Cell Vaccine Delivered Intranasally Protects Mice Against Pneumonic Plague
Uploaded by
devendersaini0 ratings0% found this document useful (0 votes)
42 views1 pagePoster presented at the 14th International Congress of Mucosal Immunology (ICMI 2009) at Boston. ICMI is recognized widely as the pre-eminent conference on mucosal immunology combining high-quality basic science with the clinical situation.
Original Title
Inactivated whole-cell vaccine delivered intranasally protects mice against pneumonic plague
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentPoster presented at the 14th International Congress of Mucosal Immunology (ICMI 2009) at Boston. ICMI is recognized widely as the pre-eminent conference on mucosal immunology combining high-quality basic science with the clinical situation.
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
42 views1 pageInactivated Whole-Cell Vaccine Delivered Intranasally Protects Mice Against Pneumonic Plague
Uploaded by
devendersainiPoster presented at the 14th International Congress of Mucosal Immunology (ICMI 2009) at Boston. ICMI is recognized widely as the pre-eminent conference on mucosal immunology combining high-quality basic science with the clinical situation.
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 1
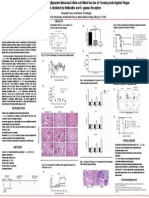
Plague is a deadly infectious disease that has killed
over 200 million people worldwide. Formalin-inactivated
Yersina pestis vaccine USP that is given by the
intramuscular route has been used for plague prevention.
However, the production of licensed Plague vaccine USP
has been discontinued due to lack of efficacy against
pneumonic plague, and its reactogenicity. We now report
that intranasal administration of paraformaldehyde-
inactivated Y. pestis CO92 is immunogenic and efficacious
in protection against pneumonic plague. Mice were
immunized intranasally with 10
8
CFU of inactivated Y.pestis
with or without 1g IL-12 on days 0 and 21. Immunized mice
were challenged intranasally on day 60 with lethal Y.pestis
CO92. The immune mice generated robust antibody
responses and showed 100% survival from lethal challenge.
This inactivated vaccine also induced BALT-like structures in
the lung that corresponded with increased levels of B-
lymphocytes, T-lymphocytes, and MHC-II+ cells in to the
lung parenchyma. Further studies are underway to
characterize the protective mechanisms.
Inactivated Whole-Cell Vaccine Delivered Intranasally Protects Mice against Pneumonic Plague
Devender Kumar and Dennis W. Metzger
Center for Immunology and Microbial Disease, Albany Medical College, Albany, NY 12208
CONCLUSIONS
We thank Michelle Wyland-O'Brien, Sharon
Salmon, and Sherie OConnell for ABSL-3
assistance.
We thank Yili Lin & Bibiana Iglesias of the AMC
Immunology Core for their help with flow cytometry
and histology.
The authors received financial support from the
DOD-ONR (Award no. N00014-06-1-1176).
ABSTRACT
INTRODUCTION
RESULTS
Fig.1. Intranasal vaccination with iYp vaccine along with
IL-12 enhances protection from pneumonic plague
Fig. 2. 10
7
CFU of intranasal iYp vaccine have limited toxicity
A. Percent body weight of mice after vaccination
B. Lung TNF- levels after vaccination
Fig. 4. IL-12 enhances the antibody titers of intranasal iYp whole-cell
vaccine
A. Serum IgG
B. Day 35 Lung antibodies
Fig. 3. Intranasal iYp vaccine induces cellular infiltration in lungs
Fig. 5. IL-12 enhances the appearance of inducible BALT-like
structures in the lungs of vaccinated mice
B. B220+ B-cells are present in Inducible BALT-like structures in
lung sections. Immunofluorescence, 40x
C. IL-12 enhances levels of B-cells, T-cells, and MHC-II + cells in
lung parenchyma after intranasal iYp vaccination. Analysis was
done by flow cytometry.
A. Lung sections, H&E staining, 40x
Plague is an exceptionally virulent and zoonotic disease.
Y. pestis is classified as a Category A select agent by the
CDC, that could to be used as a biological weapon.
Pneumonic plague has a high fatality rate even if antibiotic
treatment is started within 20 hours of infection.
Plague vaccine USP does not prevent primary pneumonic
plague in humans or mice when administered
intramuscularly.
No licensed vaccine is presently available.
Interleukin-12 (IL-12) is an extremely potent mucosal
adjuvant that enhances humoral and cell-mediated immunity.
HYPOTHESIS
Intranasal vaccination of mice with inactivated Y. pestis
vaccine adjuvanted with IL-12 will generate local and
systemic immune responses that will protect against
primary pneumonic plague.
METHODS
Vaccine: 0.25% paraformaldehyde inactivated Y. pestis
CO92 (iYp)
Animal model: BALB/c mice (n=8/gp)
Vaccine dose: 10
5
-10
8
CFU
Vaccine route: Intranasal
Vaccine volume: 30 l/mouse
Vaccination protocol:
Intranasally-administered inactivated whole-cell
Y. pestis can be used as a safe and potent
vaccine against primary pneumonic plague in
mice.
Vaccination generates robust serum and lung
antibodies.
IL-12 is an effective mucosal adjuvant for
intranasal vaccination against pneumonic plague.
Acknowledgements
Day 0 Day 21 Day 42/60
Prime Boost
Intranasal 10
4
Y.pestis CO92
Challenge
Survival
analysis
0 2 4 6 8 10 12 14
0
20
40
60
80
100
10
8
+ IL-12 10
8
PBS
0 2 4 6 8 10 12 14
0
20
40
60
80
100
10
7
+IL-12 10
7
PBS
0 2 4 6 8 10 12 14
0
20
40
60
80
100
10
6
+IL-12 10
6 PBS
0 2 4 6 8 10 12 14
0
20
40
60
80
100
10
5
+IL-12 10
5 PBS
Days post-infection
P
e
r
c
e
n
t
s
u
r
v
i
v
a
l
10
8
iYp 10
7
iYp
10
6
iYp 10
5
iYp
0 1 2 3 4 5 6 7 8 9 10 11
80
90
100
110
10
8
+ IL-12 10
8
PBS
0 1 2 3 4 5 6 7 8 9 10 11
80
90
100
110
10
7
+ IL-12 10
7
PBS
Days post-vaccination
P
e
r
c
e
n
t
W
e
i
g
h
t
10
8
iYp
10
7
iYp
1 3 5
0
500
1000
1500
2000
2500
3000
3500
4000
10
7
iYp + IL-12
10
7
iYp
PBS
Days post-immunization
T
N
F
-
(
p
g
/
m
l
)
10
7
iYp + IL-12 10
7
iYp PBS
7
5
3
D
a
y
s
p
o
s
t
-
v
a
c
c
i
n
a
t
i
o
n
0 7 14 21 28 35 42
10
100
1000
10000
100000
10
7
iYp + IL-12 10
7
iYp PBS
Days post-vaccination
S
e
r
u
m
I
g
G
T
i
t
e
r
s
Ig
M
Ig
G
Ig
G
1
Ig
G
2
a
Ig
G
2
b
Ig
G
3
Ig
A
1
10
100
1000
10000
10
7
iYp + IL-12 10
7
iYp PBS
L
u
n
g
A
n
t
i
b
o
d
y
T
i
t
e
r
s
10
7
iYp + IL-12 10
7
iYp PBS
35
14
D
a
y
s
p
o
s
t
-
v
a
c
c
i
n
a
t
i
o
n
2
8
D
a
y
s
p
o
s
t
-
v
a
c
c
i n
a
t
i o
n
10
7
iYp + IL-12 10
7
iYp PBS
B-cells T-cells MHC-II+ cells
0
1.010
6
2.010
6
3.010
6
4.010
6
5.010
6
10
7
iYp + IL-12
10
7
iYp
PBS
A
b
s
o
l u
t
e
c
e
l l n
u
m
b
e
r
s
(
L
u
n
g
)
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Results: Devender Kumar, Meiqing Shi, Laura Ristow, Woo-Yong, Lee, Paul Kubes, Jenifer Coburn, George ChaconasDocument1 pageResults: Devender Kumar, Meiqing Shi, Laura Ristow, Woo-Yong, Lee, Paul Kubes, Jenifer Coburn, George ChaconasdevendersainiNo ratings yet
- Novel Vaccines For Defense Against Pneumonic PlagueDocument43 pagesNovel Vaccines For Defense Against Pneumonic PlaguedevendersainiNo ratings yet
- Protection by Interleukin-12 Adjuvanted Intranasal Whole-Cell Killed Vaccine of Yersinia Pestis Against Plague Is Mediated by Antibodies and FC Gamma ReceptorsDocument1 pageProtection by Interleukin-12 Adjuvanted Intranasal Whole-Cell Killed Vaccine of Yersinia Pestis Against Plague Is Mediated by Antibodies and FC Gamma ReceptorsdevendersainiNo ratings yet
- Borrelia burgdorferi uses P66 adhesin, a β3-integrin ligand, for vascular transmigration in vivo.Document1 pageBorrelia burgdorferi uses P66 adhesin, a β3-integrin ligand, for vascular transmigration in vivo.devendersainiNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Evolutionary Biology NotesDocument6 pagesEvolutionary Biology NotesCleveland BrownNo ratings yet
- Modul Final Biologi Form 4 Fasa 1 2021Document60 pagesModul Final Biologi Form 4 Fasa 1 2021Manmohon Kaur100% (1)
- Enema PowepointDocument17 pagesEnema PowepointShane Lim Garcia0% (1)
- d100 Appearance/touch Taste/scent Effect MiscibilityDocument5 pagesd100 Appearance/touch Taste/scent Effect MiscibilityFoxtrot OscarNo ratings yet
- Use of Bonded Power ArmsDocument5 pagesUse of Bonded Power Armsmentacity10No ratings yet
- Horner SYndromeDocument3 pagesHorner SYndromeHendri Wijaya WangNo ratings yet
- OSCE Checklist Newborn Baby Assessment NIPEDocument3 pagesOSCE Checklist Newborn Baby Assessment NIPETauqeer Ahmed0% (1)
- LPL - PSC Rohini (DC Chowk) Shop No. 27, Ground Floor, SG Mall, Sect or - 9, DC Chowk, Rohini New Delhi - 110 DelhiDocument2 pagesLPL - PSC Rohini (DC Chowk) Shop No. 27, Ground Floor, SG Mall, Sect or - 9, DC Chowk, Rohini New Delhi - 110 DelhiSaurabh GuptaNo ratings yet
- CN 9-12Document6 pagesCN 9-12The Real UploaderNo ratings yet
- Fisier 2 Urgente Digestive EtcDocument136 pagesFisier 2 Urgente Digestive EtcDaniela PopNo ratings yet
- CT Mbbs by DR ShamolDocument197 pagesCT Mbbs by DR ShamolSiva Sandeep Chennimalai50% (2)
- Animal Welfare Act of 1998 As AmendedDocument4 pagesAnimal Welfare Act of 1998 As AmendedRex SagauinitNo ratings yet
- 101 Anti-TPO-V2.3-EN-20130731Document4 pages101 Anti-TPO-V2.3-EN-20130731Iancu Adina FloricicaNo ratings yet
- Guide HbA1c Test KitDocument18 pagesGuide HbA1c Test KitMirza KfNo ratings yet
- Modulo II Inglês - FernandaDocument76 pagesModulo II Inglês - FernandaRobison FogaçaNo ratings yet
- What Parents Should Know: About Flatfeet, Intoeing, Bent Legs and Shoes For ChildrenDocument8 pagesWhat Parents Should Know: About Flatfeet, Intoeing, Bent Legs and Shoes For ChildrenOsama HamdanNo ratings yet
- Basic Cardiac Life Support 2011Document6 pagesBasic Cardiac Life Support 2011Tashfeen Bin NazeerNo ratings yet
- Dog Forelimb Lameness ExaminationDocument9 pagesDog Forelimb Lameness ExaminationJames IrelandNo ratings yet
- Eco en UrgenciasDocument25 pagesEco en UrgenciasCristina Regueiro AraujoNo ratings yet
- English Task XI SCIENCE 3Document83 pagesEnglish Task XI SCIENCE 3Cellind WhisnyNo ratings yet
- 21 Prof Suwadi DEVIASI SEXDocument46 pages21 Prof Suwadi DEVIASI SEXdr.cintaNo ratings yet
- Balanced OcclusionDocument120 pagesBalanced Occlusionrahel sukma100% (5)
- Snapshots of A Daughter in Law RichDocument4 pagesSnapshots of A Daughter in Law RichlsacnoattNo ratings yet
- Pentavet Homeopathic Veterinary Medicine For AnorexiaDocument16 pagesPentavet Homeopathic Veterinary Medicine For AnorexiaLonely WolfNo ratings yet
- Morphology and Anatomy of EarthwormDocument30 pagesMorphology and Anatomy of Earthwormlearn easyNo ratings yet
- Healing Psoriasis by Dr. PaganoDocument3 pagesHealing Psoriasis by Dr. PaganoXavier GuarchNo ratings yet
- The Skeleton: KIN 2241b Introductory BiomechanicsDocument29 pagesThe Skeleton: KIN 2241b Introductory BiomechanicsSmiley SmilesNo ratings yet
- Editorial For December 2010 - Rare RemediesDocument4 pagesEditorial For December 2010 - Rare Remediespanniyin selvanNo ratings yet
- Gibco Neurobiology Protocols HandbookDocument110 pagesGibco Neurobiology Protocols HandbookIsaac Nicholas Notorio0% (1)
- Histology SyllabusDocument32 pagesHistology SyllabusAdrianAddieNovioDeJesusNo ratings yet