Professional Documents
Culture Documents
19 Precipitation Equilibria
Uploaded by
arvindranganathanCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
19 Precipitation Equilibria
Uploaded by
arvindranganathanCopyright:
Available Formats
A students work
(without solutions manual)
~ 10 problems/night.
Dr. Alanah Fitch
Flanner Hall 402
508-3119
afitch@luc.edu
Office Hours Th&F 2-3:30 pm
Module #19:
Precipitation Reactions
Introduction/
Context
Solution Equilibria: Solubility
In homes older than 1980 most of the plumbing
is lead pipe (Pb = plumbous). Even in newer
homes with copper pipe, solder joints are a
lead/tin alloy. Even without solder joints, many
of the faucet heads are machined with a 10-20%
lead content brass.
The limit on lead is set to be
<5 g Pb/10
9
g water
< 5 x10
--9
g Pb/g water
< 5 ppb
What to do?
Roman pipes at Pompei
Solutions:
1. Take out all plumbing
2. Place water filtration devices at
all outlets (sinks, showers, hoses).
3. Have the water department take
care of it somehow.
What do you think the average homeowner
prefers?
Coat the pipes from the inside out with
a dense impermeable quasi-permanent layer
= insoluble salt
What makes insoluble salts?
hint: same concepts as govern
what ions are or are not
spectators.
A students work
(without solutions manual)
~ 10 problems/night.
Dr. Alanah Fitch
Flanner Hall 402
508-3119
afitch@luc.edu
Office Hours Th&F 2-3:30 pm
Module #19:
Precipitation Reactions
Review Charge
Density
Energy k
q q
d
electrostatic
=
|
\
|
.
|
1 2
Its all about charge
k x
J m
C
= 899 10
9
2
.
Charge on object 1 or 2, in coulombs
Distance between the objects
d
r r
1 2
+
r
1
r
2
E k
q q
r r
el
=
+
|
\
|
.
|
1 2
1 2
Review: Module 5
Coulombs Law
Are there differences in predicted electrostatic effect?
beryllium Be2+ 2 59 0.033898305
magnesiumMg2+ 2 85 0.023529412
calcium Ca2+ 2 129.5 0.015444015
strontium Sr2+ 2 143.3333333 0.013953488
barium Ba2+ 2 149 0.013422819
Be
2+
and Mg
2+
should behave
differently
beryllium Be2+ 2 59 0.033898305
magnesiumMg2+ 2 85 0.023529412
calcium Ca2+ 2 129.5 0.015444015
strontium Sr2+ 2 143.3333333 0.013953488
barium Ba2+ 2 149 0.013422819
O
2-
and, maybe, S
2-
should behave
differently
oxide O2- -2 124.2 -0.01610306
sulfide S2- -2 170 -0.011764706
selenide Se2- -2 184 -0.010869565
telluride Te2- -2 207 -0.009661836
oxide O2- -2 124.2 -0.01610306
sulfide S2- -2 170 -0.011764706
selenide Se2- -2 184 -0.010869565
telluride Te2- -2 207 -0.009661836
fluoride F- -1 116.625 -0.008574491
chloride Cl- -1 167 -0.005988024
bromide Br- -1 182 -0.005494505
iodine I- -1 206 -0.004854369
F
-
should
behave
differently
fluoride F- -1 116.625 -0.008574491
chloride Cl- -1 167 -0.005988024
bromide Br- -1 182 -0.005494505
iodine I- -1 206 -0.004854369
H+ should
behave
differently
hydrogen D+ 1 4 0.25
lithium Li+ 1 89.66666667 0.011152416
sodium Na+ 1 127.4285714 0.007847534
potasium K+ 1 164 0.006097561
cesium Cs+ 1 192.8333333 0.005185825
hydrogen D+ 1
lithium Li+ 1
sodium Na+ 1
potasium K+ 1
cesium Cs+ 1
hydrogen D+ 1 4
lithium Li+ 1 89.66666667
sodium Na+ 1 127.4285714
potasium K+ 1 164
cesium Cs+ 1 192.8333333
hydrogen D+ 1 4 0.25
lithium Li+ 1 89.66666667 0.011152416
sodium Na+ 1 127.4285714 0.007847534
potasium K+ 1 164 0.006097561
cesium Cs+ 1 192.8333333 0.005185825
Ion Symbol Charge Radius (pm) Charge/radius
E k
q q
r r
el
=
+
|
\
|
.
|
1 2
1 2
Review: Module 5
-0.02
-0.01
0
0.01
0.02
0.03
0.04
-2.5 -2 -1.5 -1 -0.5 0 0.5 1 1.5 2 2.5
Charge on ion
C
h
a
r
g
e
/
(
a
v
e
r
a
g
e
i
o
n
i
c
r
a
d
i
u
s
)
-0.02
-0.01
0
0.01
0.02
0.03
0.04
-2.5 -2 -1.5 -1 -0.5 0 0.5 1 1.5 2 2.5
Charge on ion
C
h
a
r
g
e
/
(
a
v
e
r
a
g
e
i
o
n
i
c
r
a
d
i
u
s
)
Some of the points dont fall
Within a cluster
O
2 -
F
-
D
+
Li
+
Be
2+
Mg
2+
Review: Module 5
Charge is
Distributed
Throughout
The volume
Low charge
Density
NOT an
Alpha dog
Review: Module 5
Low Charge Density
Intermediate Charge Density
High Charge Density
Group 1 cations (1+)
NH
4
+
NO
3
-
Cl
-
SO
4
2-
OH- CO
3
2-
PO
4
3-
S
2-
Group 2 lg cations (2+)
Transition metal cations
(usually sm size 2+)
AgCl
BaSO
4
Weak Electrostatic
Interaction = Soluble
Mg(OH)
2
No Clean Socks
Who precipitates and who stays soluble?
Extremes
1. Low -Low charge density ion interactions - weak electrostatic energy Stay soluble
2. High -High charge density ion interactions - strong electrostatic energy Precipitate
3. High- Intermediate charge density ion interactions generally strong electrostatic energy precipitate
In Between
1. Low High charge density ion interactions generally weak electrostatic energy: Soluble with exceptions
2. Low Intermediate charge density ion interactions generally weak electrostatic energy: Soluble with
exceptions
3. Intermediate Intermediate charge density ion interactions generally weak electrostatic energy: soluble
with exceptions
Strong Electrostatic Interaction
results in precipitation
Oh Card me PleeeeaSe!!
Depends upon who reacts with whom?
Strong
Bases
Low Charge Density
Intermediate Charge Density
High Charge Density
NO
3
-
Cl
-
SO
4
2-
OH- CO
3
2-
PO
4
3-
S
2-
No Clean Socks
Who gives up and who holds onto a hydroxide?
Oh Card me PleeeeaSe!!
H+
STRONG acids WEAK acids
Group 2 cations (2+)
Group 1 cations (1+)
Weak bases are produced by an alternative manner
Review Acid Base Definitions: Module 6
A students work
(without solutions manual)
~ 10 problems/night.
Dr. Alanah Fitch
Flanner Hall 402
508-3119
afitch@luc.edu
Office Hours Th&F 2-3:30 pm
Module #19:
Precipitation Reactions
Solubility Product, K
sp
Mathematically Express These Concepts:
(Memorization Table is a short hand)
The smaller K, the less aquated ions, the
less soluble the material
MX H O M X
s aq aq
+ +
+
2
| || |
| || |
K
M X
MX H O
eq
aq aq
s
=
+
2
Didnt we learn
A trick about this?
| |
K K H O
so ility product eq lub
=
2
| || |
K M X
sp aq aq
=
+
| |
| || |
| |
K K H O K
M X
MX
so ility product eq eq
aq aq
s
lub
= = =
+
2
55
| |
K K H O K
so ility product eq eq lub
= =
2
55
| |
| || |
| |
| || |
K K H O K
M X
MX
M X
so ility product eq eq
aq aq
s
aq aq lub
= = = =
+
+
2
55
Solubility Constants
Numbers are determined from measuring
the amount of stuff in solution.
M
m+
X
x-
Example 1: Calculate the K
sp
of Bismuth sulfide
if there is 1.0x10
-15
mol/L of the compound
in solution at 25
o
C.
Bismuth is a post transition metal with
the electronic configuration of?:
Bi s
2
d
10
p
3
What do you think it will do to become
a cation?
Calculate the K
sp
of Bismuth sulfide
if there is 1.0x10
-15
mol/L of the compound
in solution at 25
o
C.
Lose three e
Bi s
2
d
10
p
3
Bi
3+
s
2
d
10
p
0
The electron configuration on S is:
S s
2
p
4
What will it do to get to the noble gas?
Calculate the Ksp of Bismuth sulfide
if there is 1.0x10
-15
mol/L of the compound
in solution at 25C.
Gain two e:
S s
2
p
4
S
2-
s
2
p
6
Formula?: Bi
3+
with S
2-
Bi
2
S
3
Calculate the Ksp of Bismuth sulfide
if there is 1.0x10
-15
mol/L of the compound
in solution at 25C.
Reaction?:
Now What?
What do we know/dont know/want?
Calculate the Ksp of Bismuth sulfide
if there is 1.0x10
-15
mol/L of the compound
in solution at 25C.
Bi S Bi S
s aq aq 2
3 2
2 3
+
+
| |
| |
K Bi S
sp aq
aq
=
+ 3
2
2
3
| | | | K A B
sp
a b
=
so ility of solid s x
mole
L
lub . =
10 10
15
Bi
2
S
3(solid)
2Bi
3+
aquated
+ 3S
2-
aquated
stoic 1 2 3
Init solid 0 0
Change -x +2x +3x
Equil. 1.0x10
-15
2.0x10
-15
3.0x10
-15
OJO!
Calculate the Ksp of Bismuth sulfide
if there is 1.0x10
-15
mol/L of the compound
in solution at 25C.
| |
| |
K Bi S
sp aq
aq
=
+ 3
2
2
3
| | | | K x x
sp
= 2 3
2 3
K x x x
sp
= = 4 27 108
2 3 5
( ) K x x
sp
= =
108 10 10 108 10
15
5
73
. .
| | | |
K x x
sp
=
2 0 10 30 10
15
2
15
3
. .
K x
sp
=
108 10
73
.
Or you can do it:
For an enormous list of Ksp:
http://www.northland.cc.mn.us/chemistry/solubility_products.htm
Example Calculation 2 Calculate Solubility,s, and
ion concentrations from K
sp
Candidates for water treatment for lead?
K
sp
PbF
2
4x10
-8
PbCl
2
1.6x10
-5
PbI
2
1.4x10
-8
PbSO
4
1.3x10
-8
PbCrO
4
2x10
-16
PbCO
3
1.5x10
-15
Pb(OH)
2
1.2x10
-15
PbS 7x10
-29
Pb
3
(PO
4
)
2
1x10
-54
Criteria you will
use?
Solubility = s=
amount of compound
that is soluble in
water
Do our rules clue us to Ksp values?
Pb
3
(PO
4
)
2
3Pb
2+
+ 2PO
4
3-
stoic 1 3 2
Init solid 0 0
Change -x 3x 2x
Equil? - 3x 2x
OJO!
Are we done?
No, need
[Pb
2+
]
| |
| |
K x Pb PO
sp aq
aq
= =
+
10 10
54 2
3
3
2
.
| | | | K x x x
sp
= =
10 10 3 2
54
3 2
.
K x x x x
sp
= = =
10 10 27 4 108
54 3 2 5
.
10 10
108
54
5
. x
x s
= =
9 27 10
57 5
. x x s
= =
x s x = =
62 10
12
.
| |
( ) Pb x x
aq
2 12
3 3 62 10
+
= = .
| |
Pb x
aq
2 11
18 10
+
= .
Is this below the federal standards (5 ppb)?
Yes:
| |
Pb x
aq
2 11
18 10
+
= .
18 10
1
10
207
385 10
11
3
12
. . x
molePb
L
L
gwater
gPb
mole
x
gPb
gwater
|
\
|
.
|
|
\
|
.
|
|
\
|
.
| =
385 10
10
10
385 10
10
385 10
12
9
9
3
9
3
.
.
. x
gPb
gwater
x gPb
gwater
x ppb
|
\
|
.
|
|
\
|
.
| = =
A students work
(without solutions manual)
~ 10 problems/night.
Dr. Alanah Fitch
Flanner Hall 402
508-3119
afitch@luc.edu
Office Hours Th&F 2-3:30 pm
Module #19:
Precipitation Reactions
Common Ion Effects
Will the actual amount of lead be more or
less than this value?
water
plant
PO
4
3-
Pb
3
(PO
4
)
2
LeC principle: Common ion effect
Constant flow of phosphate will
suppress lead dissociation, value should be
even lower.
( ) Pb PO Pb PO
s
aq
aq
3 4
2
2 3
3 2
,
+
+
http://www.caruschem.com/phosphate_zpoly.htm
add continuously PO
4
3
( )
Zn PO Zn P O
aq
3
3
2
3 2 6
=
K x
spZn PO
aq
3
3
2
9 0 10
33
|
\
|
.
|
= .
( ) Zincorthophosphate Zn PO
3 4
2
38605 .
s
lbs
gal
Zn P O
2 2 7
3
1
=
Zinc pyrophosphate Zn P O
2 2 7
304685 .
Name formula MM
s
lb
gal
gal
L
g
lb
mole moles
L
M =
(
= =
3 1
3785
4536
1
1
38605
09336
09336
.
.
.
.
.
P O P O H PO
P O PO H
P O PO H
H O
H O
H O
3 10
5
2 7
4
4
3
2 7
4
4
3
3 10
5
4
3
2
2
2
2
2 2
3 4
+
+
+
+ +
+
+
polyphosphate
pyrophosphate
Water quality plants use either polyphosphate
Or pyrophosphate (NOT orthophosphate)
Zn
2
P
2
O
7
2Zn
2+
2PO
4
3-
0.001
Pb
3
(PO
4
)
2
3Pb
2+
+ 2PO
4
3-
stoic 1 3 2
Init solid 0 0.001
Change -x +3x +2x
Equil? - 3x 0.001+2x
Assume 3x 0.001
Example Calculation 3Common Ion What will be the solubility of lead if a constant
stream of 0.001 M phosphate is fed through the water system?
Notice the set up here
1. Looks like accounting for H+ from water
2. Called a common ion (ion from another rx)
What will be the solubility of lead if a constant stream of 0.001 M
phosphate is fed through the water system?
| |
| |
K x Pb PO
sp
aq
= =
+
1 10
54 2
3
3
2
| | ( ) K x x
sp
= =
1 10 3 10
54
3
3
2
( ) K x x
sp
= =
1 10 27 10
54 3 6
1 10
27 10
54
6
3
x
x
x
=
37 10
50 3
. x x
=
x x =
347 10
17
.
( ) 2 2 347 10 0001
17
x x = <
. . ?
| |
| |
( )
| |
Pb x
Pb x
Pb x
aq
aq
aq
2
2 17
2 16
3
3 347 10
104 10
+
+
+
=
=
=
.
.
| |
Pb x
aq
no phosphate
2 11
18 10
+
= .
Zn
2
P
2
O
7
2Zn
2+
2PO
4
3-
0.001
Pb
3
(PO
4
)
2
3Pb
2+
+ 2PO
4
3-
stoic 1 3 2
Init solid 0 0.001
Change -x 3x 2x
Equil? - 3x 0.001+2x
Assume 3x 0.001
Wilmette, Ill.
Proprietary license
Western suburbs
Chicago Tribune, 2001
Phosphate coating
1. lowers total volume
2. Creates friction
3. Increases energy
cost
4. Lowers life span
Suburbs want
compensation from
Chicago
LeC principle: Common ion effect
( ) Pb PO Pb PO
s
aq
aq
3 4
2
2 3
3 2
,
+
+
add continuously Zn P O Zn PO H
complete
aq
aq
+ +
+ +
2 2 7
2 3
2 2 2
PO H O HPO H O
aq
aq
3
3
2
2
+
+ +
Removal of phosphate
Under acidic conditions
Should enhance solubility
Addition of phosphate should suppress solubility
( )
Pb OH Pb OH
aq aq aq
2+
+
+ +
Removal of lead
Under basic conditions
Should also enhance solubility
Can help ppt
Can prevent ppt
Hydroxide (pH) vs Carbonate
American Waterworks
Association
Pb(OH)
3
-
PbCO
3
Pb
2+
[CO
3
2-
]
Pb(OH)
2
s
pH
Solubility, s,
Depends on pH
is high at High pH, (Pb(OH)
3
-
)
and high at low pH, Pb
2+
If little dissolved carbonate
At intermediate pH
Pb(OH)
2
drops out soln
At high carbonate
PbCO
3
should drop,
BUT not very insoluble
Suppress effects
With phosphate
NOTE Scale change!
260 ppb Lead.
MARCH 20/21, 2004
0
200
400
600
800
1000
1200
0 20 40 60 80 100 120 140 160
ppb Pb in water
N
u
m
b
e
r
o
f
o
b
s
e
r
v
a
t
i
o
n
s
First Draw
0
200
400
600
800
1000
1200
0 20 40 60 80 100 120 140 160
ppb Pb in water
N
u
m
b
e
r
o
f
o
b
s
e
r
v
a
t
i
o
n
s
Second Draw
Pb pipes
Pb pipe
Cu pipe
Brass fixture
Unknown pipes
Other
Plot of amount of lead
In morning water
A students work
(without solutions manual)
~ 10 problems/night.
Dr. Alanah Fitch
Flanner Hall 402
508-3119
afitch@luc.edu
Office Hours Th&F 2-3:30 pm
Module #19:
Precipitation Reactions
Qualitative
Analysis: Intro
PbS
Muriatic acid
of or pertaining to brine
Or salt HCl
Qualitative Analysis
First known text on chemical
(as opposed of alchemical) analysis
Qualitative Analysis
M
2+
Oh hydroxide OH
-
Card Carbonate CO
3
-2
me
Plea Phosphate PO
4
3-
S Sulfide S
2-
Cl
-
NH
3
OH
-
Move back and forth between precipitate
And soluble species
( )
( )
M OH
MS
MCO
M PO
s 2
3
3 4
2
,
M X precipitate
x 2+
+
M X precipitate
nL
ML
x
x
n aq
b
2+
+
+
+
,
Preceding example was:
How to get rid of Pb
2+
by precipitation
as a phosphate
Next example:
How to bring it back into solution
a. With acid
b. With a ligand
Pb CO PbCO
aqueous
aqueous
solid
2
3
2
3
+
+
( )
Dissolving Precipitates
1. Strong acid
HCr O
H
Pb Cr O PbC O
aqueous
aqueous
solid
2 7
2
2 7
2
2 7
+
+
| +
+
+
( )
LeChatliers
Acid dissolves: chromates; carbonates, sulfides,
Dissolving Precipitates
1. Strong acid
HS
H
Pb S PbS
aqueous
aqueous
solid
+
+
| +
+
+
2 2
( )
LeChatliers
Acid dissolves: chromates, carbonates; sulfides
Dissolving Precipitates
1. Strong acid
2. Complex forming reagents (:NH
3
, :OH
-
)
Complexes
( ) ( )
Pb OH Pb OH
aq
o
s 2 2
( )
( ) ( )
Pb OH OH Pb OH
aq aq aq 3 4
2
+
( ) ( )
Pb OH OH Pb OH
aq
o
aq aq 2 3
+
( ) ( )
Pb OH OH Pb OH
aq aq aq
o +
+
2
( )
Pb OH Pb OH
aq aq aq
2+
+
+
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 2 4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 2 4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 2 4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 2 4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 2 4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
PbOH
Pb
aq
total
+
Pb
Pb
aq
total
2+
( )
Pb OH
Pb
aq
o
total
2
( )
Pb OH
Pb
aq
total
3
( )
Pb OH
Pb
aq
total
4
2
Dissolving Precipitates
1. Strong acid
2. Complex forming reagents (:OH
-
)
Pb OH Pb OH
aqueous
o
solid
( ) ( )
( ) ( ) 2 2
Neutral can result in a solid
Cation radius,CN Complex K
f
Complex K
f
CN: 4tet;6oct
Ag
+
Ag(NH
3
)
2
+
1.8x10
7
Cu
2+
71, 87 Cu(NH
3
)
4
2+
2x10
12
Zn
2+
74, 88 Zn(NH
3
)
4
2+
3.6x10
8
Zn(OH)
4
2-
3x10
14
Cd
2+
92, 109 Cd(NH3)
4
2+
2.8x10
7
Cd(OH)
4
2-
1.2x10
9
Ni
2+
Ni(NH
3
)
6
2+
9x10
8
Al
3+
67.5 Al(OH)
4
-
1x10
33
Sb
3+
90 Sb(OH)
4
-
Sn
4+
Sn(OH)
6
2-
Ligands like :NH
3
and
:OH
-
can bring various precipitates into solution
Size matters: the larger
The cation (less charge
dense) the smaller the
K
f
What do you observe?
Hold Coordination
Number constant
+
| +
+
2
3
3 2
NH
Ag NH
aqueous
aqueous
,
( )
( )
LeChatliers
Principle
Bring
Silver
Into solution
ligand: :NH
3
Ag Cl AgCl
aqueous
aqueous
solid
+
+
( )
Example 4: Calculate moles AgCl dissolved in 1 L of
6.0 M NH
3
at a temperature of 298 K
AgCl
s
[NH
3
] [Ag(NH
3
)
2
+
] [Cl
-
]
stoic n.a. 2 1 1
Init 6.0 M 0 0
Change -2x x x
Equil 6.0-2x x x
Know Dont Know Red Herring
6.0 M NH
3
s 298K
AgCl Ag Cl
solid
aqueous
aqueous
( )
+
+
( ) Ag NH Ag NH
aqueous
aq
+
+
+ 2
3 3
2
,
( ) AgCl NH Ag NH Cl
solid aq
aq
aqueous
( ) ,
,
+ +
+
2
3 3
2
reaction
K
rx
K x
sp
=
18 10
10
.
K x
f
= 17 10
7
.
( )( ) K K K x x x
reaction sp f
= = =
18 10 17 10 31 10
10 7 3
. . .
AgCl
s
[NH
3
] [Ag(NH
3
)
2
+
] [Cl
-
]
stoic n.a. 2 1 1
init 6.0 M 0 0
change -2x x x
equil 6.0-2x x x
| |
| |
31 10
6 0 2
3
2
.
.
x
x x
x
( )
| |
| |
| |
K x
Ag NH Cl
NH
reaction
= =
+
31 10
3
3
2
3
2
.
31 10
60 2
3
.
.
x
x
x
00556 60 2 . ( . ) = x x
0334 01112 . . = x x
0334 11112 . . = x
x = 0300216 .
X= moles complex
= moles AgCl dissolved
Example: Calculate moles AgCl dissolved in 1 L of
6.0 M NH
3
at a temperature of 298 K
Alanah:
Check
Moles vs
molarity
We can calculate the solubility of AgCl as a function of the ammonia concentration
| |
| |
| |
K
x x
NH x
rx
init
=
3
2
2
( )
| |
| |
| |
K x
Ag NH Cl
NH
reaction
= =
+
31 10
3
3
2
3
2
.
| |
| |
K
x
NH x
rx
init
=
3
2
( ) | |
| |
K NH x x
rx
init
3
2 =
( )| | ( )
K NH x x K
rx
init
rx 3
2 = +
( )| | ( )
K NH x K
rx
init
rx 3
1 2 = +
( )| |
( )
K NH
K
x
rx
init
rx
3
1 2 +
=
0
1
2
3
4
5
6
-4 -2 0 2 4 6 8 10 12 14
p[NH3]
s
(
M
)
AgCl becomes soluble
Around 1 M ammonia
A students work
(without solutions manual)
~ 10 problems/night.
Dr. Alanah Fitch
Flanner Hall 402
508-3119
afitch@luc.edu
Office Hours Th&F 2-3:30 pm
Module #19:
Precipitation Reactions
Qualitative Analysis:
Cl to separate Pb, Hg
2
2+
, Ag
Using pH and
complexation to
Separate Ions
For Qualitative
Analysis
Separation in lab is not always the same
As in the text.
Text starts with 6 M Cl- = pCl = -.778
The separation below
Corresponds to
What goes on in lab
Questions:
1. How does Cl
-
do the first step?
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 -1 0 1 2 3 4 5
pCl
F
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 -1 0 1 2 3 4 5
pCl
F
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 -1 0 1 2 3 4 5
pCl
F
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 -1 0 1 2 3 4 5
pCl
F
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 -1 0 1 2 3 4 5
pCl
F
r
a
c
t
i
o
n
| |
| |
o
4
4
2
PbCl
Pb
aq
aq all forms ,
| |
| |
o
1
1
+
PbCl
Pb
aq
aq all forms ,
| |
| |
o
2
2
0
PbCl
Pb
aq
aq all forms ,
| |
| |
o
3
3
PbCl
Pb
aq
aq all forms ,
| |
| |
o
o
aq
aq all forms
Pb
Pb
+ 2
,
pCl
Cl
=
=
0
1 [ ]
| | | | | | | |
Pb Pb PbCl PbCl PbCl PbCl
aq all forms
aq aq
aq
o
aq aq
,
=
(
+
(
+ + +
+ + 2
2 3 4
2
Hot water
Pb
2+
AgCl(s); Hg
2
Cl
2
(s)
Questions:
1. How does Cl
-
do the first step?
2. Why can we get Pb
2+
from Hg and Ag
with hot water?
AgCl Cl AgCl K
aqs aq aq f 2 3
2
3 , ,
+
Pb Cl PbCl K
aq aq aq f
2
1
+
+
+
PbCl Cl PbCl K
aq aw aq f
+
+
2
0
2 ,
PbCl Cl PbCl K
aq aq aq f 2
0
3 3 , ,
+
PbCl Cl PbCl K
aq aq aq f 3 4
2
4 , ,
+
PbCl
s 2
0
,
AgCl Cl AgCl K
aqs aq aq f , ,
0
2
1
2
+
Ag Cl AgCl K
aq aq aq f
+
+
,
0
1
AgCl
s ,
0
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
| |
| |
Ag
Ag
total
2+
| |
| |
AgCl
Ag
o
total
| |
| |
AgCl
Ag
total
2
| |
| |
AgCl
Ag
total
3
2
| | pCl Cl = =
2 001 ; .
For lead it was:
pCl
Cl
=
=
0
1 [ ]
AgCl Cl AgCl K
aqs aq aq f 2 3
2
3 , ,
+
Hg Cl Cl Hg Cl K
aq aq f 2 2 2 2
+
+
Pb Cl PbCl K
aq aq aq f
2
1
+
+
+
PbCl Cl PbCl K
aq aw aq f
+
+
2
0
2 ,
PbCl Cl PbCl K
aq aq aq f 2
0
3 3 , ,
+
PbCl Cl PbCl K
aq aq aq f 3 4
2
4 , ,
+
PbCl
s 2
0
,
AgCl Cl AgCl K
aqs aq aq f , ,
0
2
1
2
+
Ag Cl AgCl K
aq aq aq f
+
+
,
0
1
AgCl
s ,
0
Hg Cl
solid 2 2( )
2
2 1
Hg Cl Hg Cl K
aq aq aq f
+
+
+
Hg Cl Cl Hg Cl K
aq aq f 2 2 2 3 3
+
,
Hg Cl Cl Hg Cl K
aq aq aq f 2 3 2 4
2
4 , ,
+
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
| | pCl Cl = =
35 000035 . ; .
| |
| |
Hg
Hg
aq
total
2
2
+
+
| |
| |
Hg Cl
Hg
aq
total
2 2
0
2
,
+
| |
| |
Hg Cl
Hg
aq
total
2 3
2
,
+
| |
| |
Hg Cl
Hg
aq
total
2 4
2
2
,
+
| |
| |
Hg Cl
Hg
aq
total
2
0
2
,
+
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
f
r
a
c
t
i
o
n
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-2 0 2 4 6 8 10 12 14
pCl
F
r
a
c
t
i
o
n
Pb/Cl plot
Ag/Cl plot
Hg/Cl plot
soluble
insoluble
insoluble
Lower chloride concentration
by placing ppt in hot water.
PbCl
2
dissolves;
AgCl and Hg
2
Cl
2
remain
insoluble
Hot water
Pb
2+
AgCl(s); Hg
2
Cl
2
(s)
Questions:
1. How does Cl
-
do the first step?
2. Why can we get Pb
2+
from Hg and Ag
with hot water?
3. How do we ppt. Pb
2+
?
Pb CrO PbCrO
aqueous aqueous solid
2
4
2
4
+
+
, ,
ppt
CrO
aqueous 4
2
,
Candidates for precipitating Pb
2
from supernatent?
K
sp,
Pb Cd Zn
PbF
2
4x10
-8
- -
PbCl
2
1.6x10
-5
- -
PbI
2
1.4x10
-8
- -
PbSO
4
1.3x10
-8 - -
PbCrO
4
2x10
-16
-
_
PbCO
3
1.5x10
-15
5.5x10
-13 _
Pb(OH)
2
1.2x10
-15
6.45x10
-6
6.3x10
-17
PbS 7x10
-29
_ 1.99x10
-25
Pb
3
(PO
4
)
2
1x10
-54
_ 3.8x10
-36
Makes a very
nice, older,
chrome pigment!!
Need very insoluble species,
that doesnt bring along Cd
or Zn
S
2-
, PO
4
3-
bring Zn
2+
?
OH
-
, CO
3
2-
bring Cd
2+
Example Calculation 5. Before lead in paint was discontinued, lead
chromate was a common pigment in yellow paint. A 1.0 L solution is
prepared by mixing 0.50 mg of lead nitrate with 0.020 mg of
potassium chromate. Will a precipitate form?
( )
( )
( )
mole
gPb NO
mgPb NO
g
mg
L
x MPb
331
050
1
10
1
151 10
3
2
3
2
3
6 2
(
(
|
\
|
.
|
=
+
.
.
| || |
P Pb CrO K x
sp
= >
+ 2
4
2 16
2 10 ( ) ?
( )( ) P x x x K x
sp
= = >
151 10 1029 10 1554 10 2 10
6 7 13 16
. . . ( )
( )
mole
gK CrO
mgK CrO
g
mg
L
x MCrO
194 2
0020
1
10
1
1029 10
2 4
2 4 3
7
4
2
.
.
.
(
|
\
|
.
|
=
P K ppt forms
sp
> ;
P is like Q
NH
3
HgNH
2
Cl(s)
Ag(NH
3
)
6
+
Hot water
Questions:
1. How does Cl
-
do the first step?
2. Why can we get Pb
2+
from Hg and Ag
with hot water?
3. How do we ppt. Pb
2+
?
4. What is the purpose of NH
3
to get Ag
+
?
ppt
CrO
aqueous 4
2
,
Pb
2+
AgCl(s); Hg
2
Cl
2
(s)
( )
Ag NH AgNH
AgNH NH Ag NH
aqueous aqueous aqueous
aqueous aqueous
aqueous
+
+
+
+
+
+
3 3
3 3 3
2
AgCl Ag Cl
solid
aqueous
aqueous
( )
+
+
( ) Ag NH Ag NH
aqueous
aq
+
+
+ 2
3 3
2
,
( ) AgCl NH Ag NH Cl
solid aq
aq
aqueous
( ) ,
,
+ +
+
2
3 3
2
K x
sp
=
18 10
10
.
K x
f
= 17 10
7
.
K x
reaction
=
31 10
3
.
We did an example in which we calculated the
Solubility of AgCl in the presence of ammonia
Which number is larger?
Which has the higher solubility?
water or ammonia
( )| |
( )
K NH
K
x
rx
init
rx
3
1 2 +
=
0
1
2
3
4
5
6
-4 -2 0 2 4 6 8 10 12 14
p[NH3]
s
(
M
)
0
0.2
0.4
0.6
0.8
1
1.2
0 2 4 6 8 10
pNH3
f
r
a
c
t
i
o
n
Ag
+
Ag(NH
3
)
+
Ag(NH
3
)
2
+
Hg
2
2+
does not
form complexes
with NH
3
A students work
(without solutions manual)
~ 10 problems/night.
Dr. Alanah Fitch
Flanner Hall 402
508-3119
afitch@luc.edu
Office Hours Th&F 2-3:30 pm
Module #19:
Precipitation Reactions
Qualitative Analysis:
S to separate Cu
2+,
Hg
+
,
Bi
3+
, Cd
2+
Questions:
1. How does H
2
S do the second step?
logK
sp
S
2-
Cu
2+ -
48.5
Bi
3+
Hg
2+ -
52.7
Cd
2+
-27.0
Al
3+
Fe
3+
Cr
3+
Mn
2+ -
10.5
Zn
2+ -
24.7
Ba
2+
-
Ca
2+ -
Strong precipitators with S
2-
,
will ppt at very low [S
2-
]
Weak Precipitators with [S
2-
]
Will ppt only at higher [S
2-
]
How can we control [S2-]
Easily?
H S H HS K
aq aq aq a 2 1
+
+
HS H S K
aq aq aq a
+
+
2
2
H S H S K K
aq aq aq a a 2
2
1 2
2
+
+
0
0.2
0.4
0.6
0.8
1
0 2 4 6 8 10 12 14 16 18
pH
F
r
a
c
t
i
o
n
H
2
S
,
H
S
-
,
a
n
d
S
2
-
0
0.2
0.4
0.6
0.8
1
0 2 4 6 8 10 12 14 16 18
pH
F
r
a
c
t
i
o
n
H
2
S
,
H
S
-
,
a
n
d
S
2
-
0
0.2
0.4
0.6
0.8
1
0 2 4 6 8 10 12 14 16 18
pH
F
r
a
c
t
i
o
n
H
2
S
,
H
S
-
,
a
n
d
S
2
-
| |
| | | | | |
H S
H S HS S
aq
aq aq aq
2
2
2
+ +
| |
| | | | | |
HS
H S HS S
aq
aq aq aq
+ +
2
2
| |
| | | | | |
S
H S HS S
aq
aq aq aq
2
2
2
+ +
Control [S
2-
] through pH
Who ppts here?
Who ppt here?
Very insoluble
MS species
More soluble MS species
A students work
(without solutions manual)
~ 10 problems/night.
Dr. Alanah Fitch
Flanner Hall 402
508-3119
afitch@luc.edu
Office Hours Th&F 2-3:30 pm
Module #19:
Precipitation Reactions
Qualitative Analysis:
S to separate Zn
2+,
Co
+2
,
Mn
2+
, Ni
2+
Questions:
1. How does (NH
4
)
2
S do the first step?
2. Why is the solution basic (pH 8) in
next step?
logK
sp
S
2-
Cu
2+ -
48.5
Bi
3+
Hg
2+ -
52.7
Cd
2+
-27.0
Al
3+
Fe
3+
Cr
3+
Mn
2+ -
10.5
Zn
2+ -
24.7
Ba
2+
-
Ca
2+ -
Strong precipitators with S
2-
,
will ppt at very low [S
2-
]
Do not ppt at any value of
[S
2-
]
Intermediate: need relatively
larger values of [S
2-
] to
ppt
2M NH
4
+
; 5 MNH
3
; (NH
4
)
2
S
pH = + = 925 0397 965 . . .
Buffer pH to basic solution of 9.599
to ppt hydroxides
Ppt out sulfides
Al
3+
, Cr
3+
Fe
3+
come along
as a hydroxides
pH x
M
M
= +
log( . ) log 56 10
5
2
10
| |
| |
pH pK
A
HA
a
= + log
NH NH H K x
a 4 3
10
56 10
+
+
+ = .
0
0.2
0.4
0.6
0.8
1
1.2
4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.2
0.4
0.6
0.8
1
1.2
4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.2
0.4
0.6
0.8
1
1.2
4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.2
0.4
0.6
0.8
1
1.2
4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.2
0.4
0.6
0.8
1
1.2
4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
| |
| |
Al
Al
aq
total aq
3+
,
( )
| |
| |
Al OH
Al
aq
total aq
2+
,
( )
| |
| |
Al OH
Al
aq
total aq
2,
,
+
( )
| |
| |
Al OH
Al
aq
o
total aq
3,
,
( )
| |
| |
Al OH
Al
aq
total aq
4,
,
( ) Al OH
s
o
3,
Re-dissolve
In acid
Dissolving Precipitates
1. Strong acid
2. Complex forming reagents (:NH
3
, :OH
-
)
HCO
H
Pb CO PbCO
aqueous
aqueous
solid
3
2
3
2
3
+
+
| +
+
+
( )
LeChatliers
Acid dissolves: carbonates; sulfides, hydroxides
A students work
(without solutions manual)
~ 10 problems/night.
Dr. Alanah Fitch
Flanner Hall 402
508-3119
afitch@luc.edu
Office Hours Th&F 2-3:30 pm
Module #19:
Precipitation Reactions
Qualitative Analysis:
Separating Zn
2+,
And Al
3+
, from the sulfides
By OH complexation
Re-dissolve
In acid
Increase pH
K
f
K
sp
Ag
+
Ag(OH)
2
-
1x10
4
Ag(OH) 1.99x10
-9
Zn
2+
Zn(OH)
4
2-
4.6x10
17
Zn(OH)
2
3.16x10
-16
Mn
2+
Mn(OH)
+
2.51x10
3
Mn(OH)
2(s)
1.58x10
-13
Cr
3+
Cr(OH)
2+
6.3x10
3
Cr(OH)
3(s)
6.3x10
-31
Al
3+
Al(OH)
4
-
1x10
33
Al(OH)
3(s)
4.6x10
-33
Fe
3+
Fe(OH)
2
+
1.99x10
22
Fe(OH)
3
(s) 4x10
-38
Sn
2+
Sn(OH)
3
-
2.5x10
25
Sn(OH)
2(s)
1.2x10
-17
( )
AgOH Ag OH K x
Ag OH ag OH K x
s aq aq sp
aq aq aq f
+ =
+ =
+
+
199 10
2 1 10
9
2
4
.
,
( )
( )( ) AgOH OH Ag OH K K K x x x
s aq aq net sp f
+ = = =
2
9 4 5
199 10 1 10 199 10
,
. .
K
sp
K
f
1.99x10
-5
145
3.96x10
-10
1.28
4.6
7.96x10
-16
3.0x10
8
Which compounds can be brought into solution by raising the pH?
Example Calculation 6:
0
0.2
0.4
0.6
0.8
1
1.2
4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.2
0.4
0.6
0.8
1
1.2
4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.2
0.4
0.6
0.8
1
1.2
4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.2
0.4
0.6
0.8
1
1.2
4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
0
0.2
0.4
0.6
0.8
1
1.2
4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
| |
| |
Al
Al
aq
total aq
3+
,
( )
| |
| |
Al OH
Al
aq
total aq
2+
,
( )
| |
| |
Al OH
Al
aq
total aq
2,
,
+
( )
| |
| |
Al OH
Al
aq
o
total aq
3,
,
( )
| |
| |
Al OH
Al
aq
total aq
4,
,
( ) Al OH
s
o
3,
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 2 4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
Z
n
s
p
e
c
i
e
s
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 2 4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
Z
n
s
p
e
c
i
e
s
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 2 4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
Z
n
s
p
e
c
i
e
s
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 2 4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
Z
n
s
p
e
c
i
e
s
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 2 4 6 8 10 12 14
pH
F
r
a
c
t
i
o
n
Z
n
s
p
e
c
i
e
s
| |
| |
Zn
Zn
aq
total aq
2+
,
( )
| |
| |
Zn OH
Zn
aq
total aq
+
,
( )
| |
| |
Zn OH
Zn
aq
total aq
3,
,
( )
| |
| |
Zn OH
Zn
aq
o
total aq
2,
,
( )
| |
| |
Zn OH
Zn
aq
total aq
4
2
,
,
( ) Zn OH
s
o
2,
How do we distinguish Zn from Al?
Between Al(OH)
4
-
and Zn(OH)
4
-
only Zn has complexation with
:NH
3
Drop pH to re-ppt Al(OH)
3
, while
simultaneously forming soluble Zn(NH
3
)
4
2+
| |
| |
NH NH H K x
pH pK
A
HA
pH x
M
M
pH
a
a
4 3
10
10
56 10
56 10
3
12
9 25 0602 865
+
+ =
= +
= +
= + =
.
log
log( . ) log
. . .
3 M NH
4
+
12 M NH
3
Dissolving Precipitates
1. Strong acid
2. Complex forming reagents (:NH
3
, :OH
-
)
( )
Zn OH Zn OH
NH
Zn NH
aqueous aqueous
solid
aqueous
2
2
3
3 4
2
2
4
+
+
+
+
| +
( )
( )
( )
0
0.2
0.4
0.6
0.8
1
1.2
-2 -1 0 1 2 3 4 5 6
pNH3
f
r
a
c
t
i
o
n
Z
n
c
o
m
p
l
e
x
e
s
12 M NH
3
Zn(NH
3
)
4
2+
Zn
2+
A students work
(without solutions manual)
~ 10 problems/night.
Dr. Alanah Fitch
Flanner Hall 402
508-3119
afitch@luc.edu
Office Hours Th&F 2-3:30 pm
Module #19:
Precipitation Reactions
Qualitative Analysis:
PO
4
3-
to separate Ba
2+,
Ca
+2
, Mg
2+
CN6 r(pm) q/r K
sp
Na
+
116 0.0086 -
K
+
152 0.0065 -
NH
4
+ -
Mg
2+
86 0.023
Ca
2+
114 0.0175 Ca
3
(PO
4
)
2
1.2x10
-29
Ba
2+
149 0.013 Ba
3
(PO
4
)
2
3.4x10
-23
A students work
(without solutions manual)
~7 problems/night.
What you need
To know
Solubility
Summary Points
Complexation vs Solubility
Complexation based on electrostatic attraction
lone pairs for central cation
Size matters!; Charge matters!
Can result in multiple points of binding
Can result in charge -, o, +
Based on charge can result in precipitation
Use to manipulate and separate elements (biology too!)
Ksp = solubility product; large - # implies not soluble
Kf = formation constant; large +# implies strong binding
Calculations proceed similarly to Ka
EXCEPT STOICHIOMETRY is trickier
Ba
3
(PO
4
)
2
A students work
(without solutions manual)
~7 problems/night.
END
You might also like
- Exam 3 SolutionsDocument9 pagesExam 3 SolutionsMishka King100% (1)
- Chemistry 12 Tutorial 10 KSP CalculationsDocument12 pagesChemistry 12 Tutorial 10 KSP CalculationsrajNo ratings yet
- 222 Fall 2013 Exam 2 KeyDocument6 pages222 Fall 2013 Exam 2 KeymyNo ratings yet
- Solutions & Answers For Aieee-2012 Version - B: (Chemistry, Mathematics and Physics)Document11 pagesSolutions & Answers For Aieee-2012 Version - B: (Chemistry, Mathematics and Physics)Vivek PanchalNo ratings yet
- Chem 36: General ChemistryDocument13 pagesChem 36: General ChemistryAbdulhakeemSolimanNo ratings yet
- CLS Aipmt-18-19 XII Che Study-Package-5 SET-1 Chapter-3 PDFDocument18 pagesCLS Aipmt-18-19 XII Che Study-Package-5 SET-1 Chapter-3 PDFJayant KumarNo ratings yet
- 9701 Chemistry June 2013 SolutionDocument20 pages9701 Chemistry June 2013 SolutionSabina SabaNo ratings yet
- CH 18Document6 pagesCH 18France Mico SobrevegaNo ratings yet
- Electrochemistry Ch20bDocument13 pagesElectrochemistry Ch20bSiti Aisyah RuzelanNo ratings yet
- AP Chemistry 2010 Free-Response Questions Form B: The College BoardDocument13 pagesAP Chemistry 2010 Free-Response Questions Form B: The College BoardDharul Handri PranawaNo ratings yet
- Lection 5 (Eng) PDFDocument9 pagesLection 5 (Eng) PDFa320neoNo ratings yet
- AP Chemistry 2010 Free-Response Questions: The College BoardDocument12 pagesAP Chemistry 2010 Free-Response Questions: The College BoardDharul Handri PranawaNo ratings yet
- ChemistryDocument22 pagesChemistryGuruKPO100% (1)
- Waste Midterm PlateDocument15 pagesWaste Midterm PlateHannah Rachel UlepNo ratings yet
- Class 11 Chemistry Topperlearning Sample Paper3Document23 pagesClass 11 Chemistry Topperlearning Sample Paper3phultushiblsNo ratings yet
- WWTDocument14 pagesWWTLissa HannahNo ratings yet
- ELECTROCHEMISTRY Worksheet With AnswersDocument5 pagesELECTROCHEMISTRY Worksheet With AnswersG.D. Pranav.LaskhminarasimhanNo ratings yet
- IIT Grand Test Paper 1 Key Answers and SolutionsDocument22 pagesIIT Grand Test Paper 1 Key Answers and SolutionsRanjan PrasadNo ratings yet
- Problems in ElectrochemistryDocument9 pagesProblems in Electrochemistryrohit kohliNo ratings yet
- 2012 Y6 H2 Chem T3 CT Answers - With CommentsDocument22 pages2012 Y6 H2 Chem T3 CT Answers - With Commentsvieronic_princeNo ratings yet
- Chapter 11Document20 pagesChapter 11helloblarg100% (1)
- Electro SulDocument4 pagesElectro SulChutvinder LanduliyaNo ratings yet
- Solubility Product: Application of Equilibrium ConceptsDocument29 pagesSolubility Product: Application of Equilibrium ConceptsrajNo ratings yet
- Alum ReactionDocument5 pagesAlum Reactionyoki_triwahyudiNo ratings yet
- Solubility and EquilibriaDocument35 pagesSolubility and EquilibriaYosephine Intan AyuningtyasNo ratings yet
- 5.3.5 ChemistryDocument13 pages5.3.5 ChemistrySean Citherlet65% (17)
- 01.introduction Integrated CircuitDocument34 pages01.introduction Integrated CircuitMrinmoy DeyNo ratings yet
- Chem1102exam Nov2012Document19 pagesChem1102exam Nov2012divaaaaaaaaaNo ratings yet
- Redox Dan Electrochemistry (Kimia)Document65 pagesRedox Dan Electrochemistry (Kimia)Rocky Simon HiaNo ratings yet
- Redox Reaction and Electrochemistry 2018Document66 pagesRedox Reaction and Electrochemistry 2018Jonathan AndikaNo ratings yet
- Electrochemistry Class 12 NotesDocument53 pagesElectrochemistry Class 12 NotesGirish Arora0% (1)
- Soal (1) (Repaired)Document9 pagesSoal (1) (Repaired)Inda AlwanNo ratings yet
- Chemistry 222 Exam 4: Chapters 11, 13, 14 (16ptsDocument7 pagesChemistry 222 Exam 4: Chapters 11, 13, 14 (16ptsSayNo ratings yet
- Redox TitrationDocument14 pagesRedox Titrationnorsiah100% (2)
- Circuits and ElektroniksDocument415 pagesCircuits and ElektroniksjftheoretNo ratings yet
- 12878anil Samples Paper 2012 ExamDocument15 pages12878anil Samples Paper 2012 Examamit34521No ratings yet
- Chapter20 Solubility Product ConstantDocument32 pagesChapter20 Solubility Product ConstantJohn Mark OsiasNo ratings yet
- Tutorial 3 Electrochemistry - AnswersDocument10 pagesTutorial 3 Electrochemistry - AnswerssgarrabNo ratings yet
- Water Softening Process OverviewDocument5 pagesWater Softening Process OverviewXherine Bico CordialNo ratings yet
- Stoichiometry: Unit: 3Document5 pagesStoichiometry: Unit: 3Premangshu GhoshalNo ratings yet
- JEE MAINS - Test 10 - Solution Notes (Chemistry) - JEE Mains - Test 10 Solution Notes (Chemistry)Document33 pagesJEE MAINS - Test 10 - Solution Notes (Chemistry) - JEE Mains - Test 10 Solution Notes (Chemistry)Mohit SuaradkarNo ratings yet
- Electrochemistry Chapter 17 - 1Document69 pagesElectrochemistry Chapter 17 - 1Priscilla GarzaNo ratings yet
- CHEMISTRY REVISIONDocument10 pagesCHEMISTRY REVISIONaNo ratings yet
- MARKING SCHEME (2021-22) Term - Ii Chemistry Theory (043) Set ADocument5 pagesMARKING SCHEME (2021-22) Term - Ii Chemistry Theory (043) Set ASyed ShammazNo ratings yet
- EXERCICE: Carbonate Equilibrium: Molar Masses: CaDocument5 pagesEXERCICE: Carbonate Equilibrium: Molar Masses: CaGEMPFNo ratings yet
- ERDMANN EXAM 3 SPRING 2019 CH 117 REVIEWDocument6 pagesERDMANN EXAM 3 SPRING 2019 CH 117 REVIEWharlow6winfield6adamNo ratings yet
- PH.D Chemistry 2015Document27 pagesPH.D Chemistry 2015alienNo ratings yet
- Electrochemistry: Applications of RedoxDocument40 pagesElectrochemistry: Applications of Redoxcatsathish1No ratings yet
- Solubility of Caso: Major Concepts and Learning GoalsDocument6 pagesSolubility of Caso: Major Concepts and Learning GoalsNacorn PanchanawapornNo ratings yet
- CHEM 301 Assignment #1Document17 pagesCHEM 301 Assignment #1san toryuNo ratings yet
- 08-JEE-Adv Grand Test 08 Solutions (P 2)Document13 pages08-JEE-Adv Grand Test 08 Solutions (P 2)Ranjan PrasadNo ratings yet
- Assign 1 2016 SolutionsDocument17 pagesAssign 1 2016 SolutionsIkhsan RifqiNo ratings yet
- Electrochemistry Worksheet 2: Done in FigDocument8 pagesElectrochemistry Worksheet 2: Done in Figrezwanur rahmanNo ratings yet
- Class XII - All India Chemistry - Set-2: Alkyl Halide Sodium Alkoxide EtherDocument4 pagesClass XII - All India Chemistry - Set-2: Alkyl Halide Sodium Alkoxide EtherShashank ShekharNo ratings yet
- NOTES ElectrochemistryDocument30 pagesNOTES ElectrochemistryAlexander LeeNo ratings yet
- PoopDocument11 pagesPoopkurt2011100% (1)
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- 150 Interview QuestionsDocument167 pages150 Interview QuestionsPriyanka Kumari100% (2)
- Sentence Types: There Are 3 Types of Sentences. Can You Name Them All?Document10 pagesSentence Types: There Are 3 Types of Sentences. Can You Name Them All?arvindranganathanNo ratings yet
- Seasons of The Year MatchingDocument1 pageSeasons of The Year Matchingarvindranganathan100% (1)
- Kids Print SeasonsDocument1 pageKids Print SeasonsarvindranganathanNo ratings yet
- Astronomy Lesson 4Document6 pagesAstronomy Lesson 4arvindranganathanNo ratings yet
- Seasons EarthSunDistDocument10 pagesSeasons EarthSunDistarvindranganathanNo ratings yet
- Seasons Arnolds Apple TreeDocument7 pagesSeasons Arnolds Apple Treearvindranganathan100% (1)
- Look Out There PowerpointDocument11 pagesLook Out There PowerpointarvindranganathanNo ratings yet
- Outerspace DefinitionsDocument1 pageOuterspace DefinitionsarvindranganathanNo ratings yet
- Maths ActivitiesDocument85 pagesMaths ActivitiesarvindranganathanNo ratings yet
- Constellation Work BookDocument15 pagesConstellation Work BookarvindranganathanNo ratings yet
- Instructors GuideDocument55 pagesInstructors GuideskypaulNo ratings yet
- Animals Which Kill Other AnimalsDocument1 pageAnimals Which Kill Other AnimalsarvindranganathanNo ratings yet
- Worksheet Rotation, Revolution, Tilt and SeasonsDocument2 pagesWorksheet Rotation, Revolution, Tilt and SeasonsarvindranganathanNo ratings yet
- 35 Solar System ReasonsForTheSeasonsDocument4 pages35 Solar System ReasonsForTheSeasonsarvindranganathanNo ratings yet
- Voice and Accent Training MaterialDocument30 pagesVoice and Accent Training MaterialAnwar GaffNo ratings yet
- Voyage G58 L1Document42 pagesVoyage G58 L1arvindranganathanNo ratings yet
- Planet Sword SearchDocument1 pagePlanet Sword SearcharvindranganathanNo ratings yet
- What Is A Group DiscussionDocument10 pagesWhat Is A Group Discussionvinodh76No ratings yet
- Outerspace DefinitionsDocument1 pageOuterspace DefinitionsarvindranganathanNo ratings yet
- World War IDocument16 pagesWorld War IarvindranganathanNo ratings yet
- 4 Seasons WheelDocument2 pages4 Seasons WheelarvindranganathanNo ratings yet
- World War IIDocument6 pagesWorld War IIarvindranganathanNo ratings yet
- What Is ISO 17712Document9 pagesWhat Is ISO 17712arvindranganathanNo ratings yet
- Types of Informational MapsDocument8 pagesTypes of Informational MapsarvindranganathanNo ratings yet
- Training Needs AnalysisDocument11 pagesTraining Needs AnalysisarvindranganathanNo ratings yet
- Types of Informational MapsDocument8 pagesTypes of Informational MapsarvindranganathanNo ratings yet
- The Number of Countries in TheDocument14 pagesThe Number of Countries in ThearvindranganathanNo ratings yet
- Thankyou LetterDocument18 pagesThankyou LetterarvindranganathanNo ratings yet
- Absorption Refrigeration SystemsDocument33 pagesAbsorption Refrigeration SystemsAdedire FisayoNo ratings yet
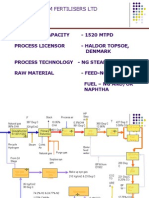
- KRIBHCO SHYAM FERTILISERS LTD AMMONIA PROCESS OVERVIEWDocument51 pagesKRIBHCO SHYAM FERTILISERS LTD AMMONIA PROCESS OVERVIEWSaad Khan89% (9)
- Stainless Steel Evaporative Condenser / Fluid-Cooler: Installation and Operation ManualDocument34 pagesStainless Steel Evaporative Condenser / Fluid-Cooler: Installation and Operation Manualjose luisNo ratings yet
- Intro To Atoms Moles and Stoichiometry: As Level Chemistry Test Name: Class: TeacherDocument8 pagesIntro To Atoms Moles and Stoichiometry: As Level Chemistry Test Name: Class: TeacherMatthew James PopeNo ratings yet
- AmoniaDocument35 pagesAmonianoelia cossio0% (1)
- Textile Dyeing Effluent Treatment Using Solar Photo-Fenton ProcessDocument84 pagesTextile Dyeing Effluent Treatment Using Solar Photo-Fenton ProcessGUILLERMO ALEJANDRO MARTINEZ LOPEZNo ratings yet
- Simulation of A Reactor Considering The Stamicarbon, Snamprogetti, and Toyo Patentes For Obtaining UreaDocument7 pagesSimulation of A Reactor Considering The Stamicarbon, Snamprogetti, and Toyo Patentes For Obtaining UreaMISHEL ROSARIO ROMERO MARAVINo ratings yet
- 14 Science ST Edwards School 2014Document19 pages14 Science ST Edwards School 2014ryanbatiNo ratings yet
- HL Paper 2: Urea synthesis and analysisDocument36 pagesHL Paper 2: Urea synthesis and analysisSharon ChanNo ratings yet
- Thai Natural LatexDocument3 pagesThai Natural LatexnuoyaprecisionNo ratings yet
- Method for producing isopropylamine via sectional temperature controlDocument8 pagesMethod for producing isopropylamine via sectional temperature controlMariana PopescuNo ratings yet
- Pixl Knowit!: Gcse ChemistryDocument80 pagesPixl Knowit!: Gcse ChemistryBenjamin WatsonNo ratings yet
- Improved Gas Sensors Based on Novel Semiconducting Metal OxidesDocument5 pagesImproved Gas Sensors Based on Novel Semiconducting Metal OxidesadityaNo ratings yet
- 9701 - w18 - Ci - 31 (FEB 2020) PDFDocument8 pages9701 - w18 - Ci - 31 (FEB 2020) PDFMuhammad Andi TanriNo ratings yet
- Pearson 2008Document7 pagesPearson 2008Carlos DelgadoNo ratings yet
- 21-01-2024 - SR - Super60 - Elite, Target & LIIT-BTs - Jee-Main-GTM-15 - KEY & Sol'SDocument16 pages21-01-2024 - SR - Super60 - Elite, Target & LIIT-BTs - Jee-Main-GTM-15 - KEY & Sol'SAshwina JaikrishnanNo ratings yet
- Limits On The Space-Time Variations of Fundamental Constants 1307.8266Document6 pagesLimits On The Space-Time Variations of Fundamental Constants 1307.8266forizslNo ratings yet
- Wastewater Treatment Systems ModuleDocument203 pagesWastewater Treatment Systems Moduleetayhailu100% (8)
- Diffusion in Gases and LiquidsDocument40 pagesDiffusion in Gases and LiquidsOyasor Ikhapo AnthonyNo ratings yet
- Ammonium Chloride: Safety Data SheetDocument8 pagesAmmonium Chloride: Safety Data SheetEnam HaqNo ratings yet
- Compressor NomenclatureDocument4 pagesCompressor NomenclatureMuhammadUsmanSaeedNo ratings yet
- Heat of Formation of Ammonium CarbamateDocument7 pagesHeat of Formation of Ammonium CarbamateNegruskoNo ratings yet
- Unit 3. Solutions To Sample ProblemsDocument8 pagesUnit 3. Solutions To Sample ProblemsFat PatNo ratings yet
- Process 2Document5 pagesProcess 2Mika PelagioNo ratings yet
- 2021 IFA Medium Term Outlook Public SummaryDocument9 pages2021 IFA Medium Term Outlook Public SummaryMaria Jezreel BernaldezNo ratings yet
- Aldehydes & Ketones: Properties, Preparation and ReactionsDocument45 pagesAldehydes & Ketones: Properties, Preparation and ReactionsShivam GuptaNo ratings yet
- CD4855 - Ammonia - Urea - Markets - in - Latin - America - January - 2009 - (UPDATED - 9th - Jan) - Final BRITISH 02 PDFDocument60 pagesCD4855 - Ammonia - Urea - Markets - in - Latin - America - January - 2009 - (UPDATED - 9th - Jan) - Final BRITISH 02 PDFgabydel74No ratings yet
- Answer: 660 GRAMSDocument16 pagesAnswer: 660 GRAMSakshayatejomurthulaNo ratings yet
- F&EI Calculation WorkbookDocument58 pagesF&EI Calculation WorkbookMufti Sinergi SolusiNo ratings yet
- Ammonia Absorption Process GuideDocument35 pagesAmmonia Absorption Process GuideMuzzy VoraNo ratings yet