Professional Documents
Culture Documents
UII Gram Pos Spore-Form
Uploaded by
Dita Wahyu RahmanCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
UII Gram Pos Spore-Form
Uploaded by
Dita Wahyu RahmanCopyright:
Available Formats
Spore Forming
Gram-positive Bacilli
Titik Nuryastuti
Microbiology Department,
Fac. of Medicine, UGM
Spores
Why do bacteria produce spores?
Survival
Classification
Definition = a resting cell, highly
resistant to dessication, heat, and
chemical agents; when returned to
favourable conditions bacteria re-
activated, the spores germinate to
produce single vegetative cells.
SF Bacteria- Bacillus
Aerobic, G+ rods in chains, spores are
located in center of the non-motile bacilli
Found in soil, water, air and vegetation
Spores are viable for decades.
B. cereus produce enterotoxin and cause
food poisoning.
B. anthracis infection in human through
injured skin (cutaneous anthrax), mucous
membranes (GI anthrax), or inhalation of
spores into lung.
Bacillus anthracis
Spores germinate in the tissue of entry,
and growth of vegetative organisms
result in formation of a gelatinous
oedema and congestion.
Spread via lymphatics to bloodstream
and multiply freely in blood and tissues.
Capsulated, poly-D-glutamic acid
capsule is antiphagocytic
SF Bacteria- Bacillus
Anthrax toxin is made up of three proteins:
Protective antigen (PA), edema factor (EF) and lethal
factor (LF).
Clinical finding :
Cutaneous Anthrax(malignant pustule):
Generally occurs on exposed surfaces of the arms,
face and neck through wound contamination by the
spores of the organism. About 95% of the cases with
amortality rate 20% .
Inhalation Anthrax(wool sorter disease):
About 5% of the cases with 85-90% mortality.
Treatment: ciprofloxacin, penicillin G along with
gentamicin and streptomycin.
SF Bacteria- Bacillus
SF Bacteria-Bacillus
Lab diagnosis :
Gram staining
Culture on Blood agar
Speciment :
Fluid, pus, blood, sputum
SF Bacteria - Clostridium
Anaerobic, G+, motile rods
Their natural habitat is the soil or the
intestinal tract of human and animals,
where they live as saprophytes
Found in soil, animal faeces.
Spores is placed centrally, subterminally
or terminally; most species are motile
with flagella.
SF Bacteria - Clostridium
Many decompose proteins of form
toxins, some do both
Among the pathogens are the
organisms causing botulism, tetanus,
gas gangrene, and pseudomembranous
colitis.
C. botulism, C. tetani, C. perfringens, C.
difficile
SF Bacteria - Clostridium
Many form colonies with a zone of
haemolysis on blood agar. C perfringens
typically produce multiple zones of
haemolysis around colonies.
Identification
In most species, the spores are located
centrally, subterminally or terminally.
Most species of Clostridia are motile
with peritrichous flagella
Clostridium
Epidemiology
Ubiquitous
Present in soil, water, sewage
Normal flora in GI tracts of animals and
humans
Pathogenesis
Spore formation
resistant to heat, dessication, and disinfectants
can survive for years in adverse environments
Rapid growth in oxygen deprived, nutritionally
enriched environment
Toxin elaboration (histolytic toxins, enterotoxins,
neurotoxins)
Clostridium botulinum
Epidemiology
Commonly isolated in soil and water
Human disease associated with botulinum toxin A, B,
E, F
Pathogenesis
Blocks neurotransmission at peripheral cholinergic
synapses
Prevents release of acetylcholine, resulting in muscle
relaxation
Recovery depends upon regeneration of nerve
endings
SF Bacteria C.botulinum
C botulinum causes botulism
-Distinguished by antigenic type of toxin
Spores are resistant to 100C for many hours,
diminished at acid pH or high salt.
Toxin - 7 antigenic varieties (A G). A, B, E
(F) mainly harmful to human.
Botulinum toxin is absorbed from gut and
binds to receptors of presynaptic nervous
system and cranial nerves.
Lethal dose to human 1-2 g.
SF Bacteria - Clostridium
Pathogenesis
Most cases, through ingestion of uncooked
food.
Toxin acts by blocking release of acetylcholine
at synapses and neuromuscular junctions
flacid paralysis.
Symptoms such as visual disturbances,
inability to swallow, speech problem; seldom
with no apparent GI symptoms; no fever.
Botulism
Clinical Syndromes
Foodborne botulism
Associated with consumption of preformed toxin
Home-canned foods (toxin A, B)
Preserved fish (toxin E)
Onset of symptoms 1-2 days
Blurred vision vision, dilated pupils, dry mouth, constipation
Bilateral descending weakness of peripheral muscles; death
related to
respiratory failure
Infant botulism
Consumption of foods contaminated with botulinum spores
6-10% of syrups or honeys
Disease associated with neurotoxin produced in vivo
Onset of symptoms in 3-10 days
Wound botulism (skin popping)
Asymptomatic adult carriage
Botulism: Treatment
Treatment
Supportive care
Elimination of organism from GI tract
Gastric lavage
Metronidazole or penicillin
Botulinum Immunoglobulin (BIG): pooled plasma from adults
immunized with pentavalent (ABCDE) botulinum toxoid
Trivalent equine Immunoglobulin (ABE)
Prevention
Prevention of spore germination (Storage <4C, high sugar
content, acid PH)
Destruction of preformed toxin (20 min at 80C)
SF Bacteria - Clostridium
floppy baby = infant botulism. C botulinum
spores in babies food.
Treatment antitoxins raised in horses.
Trivalent (A, B, E) antitoxin must be promptly
administered intravenously with precautions;
plus adequate ventilations.
Clostridium tetani
Epidemiology
Spores found in most soils, GI tracts of animals
Disease in un-vaccinated or inadequately immunized
Disease does not induce immunity
Pathogenesis
Spore inoculated into wound
Tetanospasmin
Heat-labile neurotoxin
Retrograde axonal transport to CNS
Blocks release of inhibitory neurotransmitters (eg. GABA) into
synapses, allowing excitatory synapses to be unregulated. This
results in muscle spasms
Binding is irreversible
Tetanolysin
Oxygen labile hemolysin, unclear clinical significance
SF Bacteria C.tetani
Clostridium tetani cause tetanus.
Distinguishable by specific flagellar antigens.
Pathogenesis: Wound contamination, not
an invasive organism. The toxins released
from vegetative cells reaches the CNS and
rapidly becomes fixed to receptors in the
spinal cord and brain stem and exerts their
action.
C. tetani
Toxins: Tetanospasmin
binds to receptors on the presynaptic
membranes of motor neurons.
Clinical Findings: Incubation period:
4-5 days to many weeks. The disease
is chacterized by tonic contraction of
voluntary muscles.
Tetanus
Treatment
Debridement of wound
Metronidazole
Tetanus immunoglobulin
Prevention
Vaccination with a series of 3 tetanus
toxoid
Booster dose every 10 years
Clostridium perfringens
Epidemiology
GI tract of humans and animals
Type A responsible for most human infections, is widely distributed in
soil and water contaminated with feces
Type B-E do not survive in soil but colonize the intestinal tracts of
animals and occasionally humans
Pathogenesis
-toxin: lecithinase (phospholipase C) that lyses erythrocytes,
platelets and endothelial cells resulting in increased vascular
permeability and hemolysis
-toxin: necrotizing activity
Enterotoxin: binds to brush borders and disrupts small intestinal
transport resulting in increased membrane permeability
Clinical manifestations
Self-limited gastroenteritis
Soft tissue infections: cellulitis, fascitis or myonecrosis (gas
gangrene)
C. perfringens
Many different- toxin producing clostridia can
produce invasive infections(including myonecrosis
and gas gangrene) if introduced into damaged tissue.
About 30 species of clostridia may produce such an
infection, but the most common in invasive disease is
C. perfringens(90%). An enterotoxin of C.
perfringens is a common cause of food poisoning.
Toxins: produce different types of toxins and
enzymes that result in spreading infection. They
have lethal, necrotizing, and hemolytic properties.
Pathogenesis: Wound contamination.
Clinical Findings: Infection spreads in 1-3 days.
Crepitation in subcutaneous tissue and muscle, fever,
tissue necrosis, hemolytic anemia, severe toxemia
and death.
Clostridial soft tissue infections
Crepitant cellulitis
Fascitis
Myonecrosis
Clostridial myonecrosis
Clinical course
Symptoms begin 1-4 days after inoculation and
progresses rapidly to extensive muscle necrosis and
shock
Local area with marked pain, swelling, serosanguinous
discharge, bullae, slight crepitance
May be associated with increased CPK
Treatment
Surgical debridement
Antibiotics
Hyperbaric oxygen
C. difficile
Pseudomembranous colitis (Antibiotic
Associated Diarrhea)
Clostridium difficile
Epidemiology
Endogenous infection
Colonizes GI tract in 5% healthy individuals
Antibiotic exposure associated with overgrowth of C. difficile
Cephalosporins, clindamycin, ampicllin/amoxicillin
Other contributing factors: agents altering GI motility,
surgery, age, underlying illness
Exogenous infection
Spores detected in hospital rooms of infected patients
Pathogenesis
Enterotoxin (toxin A)
produces chemotaxis, induces cytokine production and
hypersecretion of fluid, development of hemorrhagic
necrosis
Cytotoxin (toxin B)
Induces polymerization of actin with loss of cellular
cytoskeleton
C. difficile colitis
Clinical syndromes
Asymptomatic colonization
Antibiotic-associated diarrhea
Pseudomembranous colitis
Diagnosis
Isolation of toxin
Culture
Treatment
Discontinue antibiotics
Metronidazole or oral vancomycin
Pooled human IVIG for severe disease
Probiotics (saccharomyces boulardii)
New drugs (nitazoxanide, tolevamer)
Relapse in 20-30% (spores are resistant)
The Genus
Clostridium
Left. Stained pus from a mixed anaerobic
infection. At least three different clostridia
are apparent. Right. Electron micrograph of
Clostridium tetani
C. botulinum
C.perfringens
C. tetani
C. difficile
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Kuliah 1 HHD HipertensiDocument41 pagesKuliah 1 HHD HipertensiDita Wahyu RahmanNo ratings yet
- Kuliah 1 HHD HipertensiDocument41 pagesKuliah 1 HHD HipertensiDita Wahyu RahmanNo ratings yet
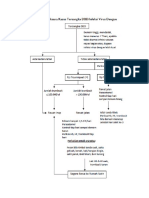
- Pathway DF DHFDocument4 pagesPathway DF DHFDita Wahyu RahmanNo ratings yet
- Kuliah 1 Hiv, Aids, OpportDocument61 pagesKuliah 1 Hiv, Aids, OpportDita Wahyu RahmanNo ratings yet
- Kuliah 1 HHD HipertensiDocument41 pagesKuliah 1 HHD HipertensiDita Wahyu RahmanNo ratings yet
- Islam Perspective On Heart DiseaseDocument39 pagesIslam Perspective On Heart DiseaseDita Wahyu RahmanNo ratings yet
- The Curriculum PhysicsDocument4 pagesThe Curriculum PhysicsDita Wahyu RahmanNo ratings yet
- Ce 15403Document18 pagesCe 15403Dita Wahyu RahmanNo ratings yet
- Stroke 2010 Hassan 1673 8Document7 pagesStroke 2010 Hassan 1673 8Dita Wahyu RahmanNo ratings yet
- Stroke 2010 Bath 732 8Document8 pagesStroke 2010 Bath 732 8Dita Wahyu RahmanNo ratings yet
- Stroke 2010 Bath 732 8Document8 pagesStroke 2010 Bath 732 8Dita Wahyu RahmanNo ratings yet
- CDC-wfa Boys 2 To 20 YearsDocument1 pageCDC-wfa Boys 2 To 20 YearsDita Wahyu RahmanNo ratings yet
- CDC-wfa Girls Birth To 36 MonthDocument1 pageCDC-wfa Girls Birth To 36 MonthDita Wahyu RahmanNo ratings yet
- Growth Chart For Boys Birth To 36 MonthsDocument2 pagesGrowth Chart For Boys Birth To 36 MonthsCarlos TejedaNo ratings yet
- Topic Tree EmergenciesDocument1 pageTopic Tree EmergenciesDita Wahyu RahmanNo ratings yet
- Kuliah 1 HHD HipertensiDocument41 pagesKuliah 1 HHD HipertensiDita Wahyu RahmanNo ratings yet
- Kuliah 1 HHD HipertensiDocument41 pagesKuliah 1 HHD HipertensiDita Wahyu RahmanNo ratings yet
- Brain Death AdultsDocument4 pagesBrain Death AdultsTcacolate Telsa ElizsabethNo ratings yet
- Evidence Based Medicine 0705Document32 pagesEvidence Based Medicine 0705Dita Wahyu RahmanNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Evaluation of Diarrhea in ChildrenDocument21 pagesEvaluation of Diarrhea in ChildrenDagnachew kasayeNo ratings yet
- Halosil Brochure Web 030717Document6 pagesHalosil Brochure Web 030717duna tarimaNo ratings yet
- Infectious DiseasesDocument37 pagesInfectious Diseasespolaris_027No ratings yet
- Power Point Environmental CleaningDocument60 pagesPower Point Environmental CleaningPrapitta AnindyaNo ratings yet
- Zithromax Powder For Oral Suspension - (EMC) Print FriendlyDocument12 pagesZithromax Powder For Oral Suspension - (EMC) Print Friendlyle minh leNo ratings yet
- Infection in Critical CareDocument34 pagesInfection in Critical CareSuresh Kumar BansalNo ratings yet
- Cdi and FMT Risks and BenefitsDocument5 pagesCdi and FMT Risks and Benefitsapi-426734065No ratings yet
- 2019 MUSE Conference - Educational Presentations - Rev May 2 2019Document81 pages2019 MUSE Conference - Educational Presentations - Rev May 2 2019Michele LambertNo ratings yet
- CAST Probiotics Issue Paper FINAL144Document20 pagesCAST Probiotics Issue Paper FINAL144Arup ChakrabortyNo ratings yet
- Bugs & DrugsDocument33 pagesBugs & Drugsveronica100% (1)
- Brook2005 PDFDocument9 pagesBrook2005 PDFNunoGonçalvesNo ratings yet
- PosterDocument45 pagesPosterRoxana Alexandra BogosNo ratings yet
- DeclomycineDocument14 pagesDeclomycineRamanasarmaNo ratings yet
- BacteriaDocument13 pagesBacteriathzone1986No ratings yet
- 2019 MUSE Conference - Educational Presentations - Rev March 19 2019Document52 pages2019 MUSE Conference - Educational Presentations - Rev March 19 2019Michele LambertNo ratings yet
- Med Surg Pico-2Document11 pagesMed Surg Pico-2Jason Kennedy100% (1)
- Clostridium Difficile Infection and Fecal BacteriotherapyDocument9 pagesClostridium Difficile Infection and Fecal BacteriotherapyAnonymous nEC4alrPjGNo ratings yet
- Microbiology UW New by FinalstepsDocument41 pagesMicrobiology UW New by Finalstepsxinfox11No ratings yet
- Environmental Hygiene - The Importance of Process, Product and Practice PDFDocument18 pagesEnvironmental Hygiene - The Importance of Process, Product and Practice PDFAnsh KunalNo ratings yet
- 019537s082 020780s040lbl PDFDocument43 pages019537s082 020780s040lbl PDFShahab Ud DinNo ratings yet
- Review 2Document12 pagesReview 2Christine Rubio100% (1)
- End State Renal Final Case Study MNTDocument18 pagesEnd State Renal Final Case Study MNTapi-242547654No ratings yet
- Fishbone CDiff - Revised 0310Document1 pageFishbone CDiff - Revised 0310Anonymous A5wp71HzNo ratings yet
- Antibiotics in ICUDocument63 pagesAntibiotics in ICUsayantanNo ratings yet
- B. Belingon - Notes From Case Session Slides, Anna's Notes (Dr. Esterl), Becky's Notes (Dr. Nguyen)Document11 pagesB. Belingon - Notes From Case Session Slides, Anna's Notes (Dr. Esterl), Becky's Notes (Dr. Nguyen)lizzy596No ratings yet
- MicrobiologyDocument1,299 pagesMicrobiologyBenjamin Agbonze100% (1)
- Internal Medicine Clinical Practice Guidelines 2018 Midyear Review PDFDocument17 pagesInternal Medicine Clinical Practice Guidelines 2018 Midyear Review PDFveerrajuNo ratings yet
- Diare PDFDocument23 pagesDiare PDFGangsar DamaiNo ratings yet
- 2019 MUSE Conference - Educational Presentations - Rev March 21 2019Document60 pages2019 MUSE Conference - Educational Presentations - Rev March 21 2019Michele LambertNo ratings yet
- Micro Bio Disease ListDocument168 pagesMicro Bio Disease Listspiff spacemanNo ratings yet