Professional Documents
Culture Documents
PH Indicator
Uploaded by
Adil Amin0 ratings0% found this document useful (0 votes)
161 views25 pagesph
Original Title
Ph Indicator
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentph
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
161 views25 pagesPH Indicator
Uploaded by
Adil Aminph
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 25
pH INDICATOR
The compounds (mostly organic) which
change their color when pH of the solution
changes
Indicators are used to;
1) Check the pH of the solution.
2) Indicate the end-point i.e. completion of
the reaction.
At 25 C, considered the standard temperature, the pH
value of a neutral solution is 7.0. Solutions with a pH value
below 7.0 are considered acidic, whereas solutions with pH
value above 7.0 are basic (alkaline). As most naturally
occurring organic compounds are weak carboxylic acids and
amines, pH indicators find many applications in biology and
analytical chemistry.
Types of indicators
The choice of the indicator depends upon the nature of
the reaction. There are three basic types of indicators.
1) Acid- base indicator
2) Redox indicators
3) Precipitation indicators
For the quantitative analysis of metal cations, the use of
complexometric indicators is preferred.
Acid Base indicators:
These are the organic compounds which are used in acid
base titrations. Acid - base indicators, respond to a change
in the hydrogen ion concentration. Most of the indicators
are themselves weak acids other are weak bases which
responded to change hydroxyl ion concentration.
Color Blue Litmus Red Litmus
Acid turns red stays same
Base stays same turns blue
The most common indicator is found on "litmus" paper. It is red below pH 4.5 and blue above pH 8.2.
An indicator is usually some weak organic acid or base dye that
changes colors at definite pH values. The weak acid form (HIn) will
have one color and the weak acid negative ion (In
-
) will have a
different color. The weak acid equilibrium is:
HIn --> H+ + In
-
For phenolphthalein: pH 8.2 = colorless; pH 10 = red
For bromophenol blue: pH 3 = yellow; pH 4.6 = blue
Magic Pitcher Demonstration:
Phenolphthalein is an indicator of acids (colorless) and bases (pink).
Sodium hydroxide is a base, and it was in the pitcher at the
beginning, so when added to the phenolphthalein in beakers 2 and
4, it turned pink (top half of the graphic).
Explanation:
Equilibrium: HIn --> H
+
+ In
-
colorless pink
The equilibrium shifts right, HIn decreases, and In
-
increases. As the
pH increase between 8.2 to 10.0 the color becomes red because of
the equilibrium shifts to form mostly In
-
ions.
The third beaker has only the NaOH but no phenolphthalein, so it
remained colorless. The first beaker contain acetic acid and is skipped
over at first.
After pouring beakers 2, 3, 4 back into the pitcher it give a pink solution.
Bottom half of the graphic: When the pitcher is then poured back into
beakers 2, 3, 4 it is a pink solution.
In the first beaker, a strange thing happens in that the pink solution
coming out of the pitcher now changes to colorless. This happens
because the first beaker contains some vinegar or acetic acid which
neutralizes the NaOH, and changes the solution from basic to acidic.
Under acidic conditions, the phenolphthalein indicator is colorless.
Neutralization: HC
2
H
3
O
2
+ NaOH --> Na(C
2
H
3
O
2
) + HOH
The simplified reaction is:
H
+
+ OH
-
--> HOH
As OH
-
ions are added, they are consumed by the excess of acid
already in the beaker as expressed in the above equation. The
hydroxide ions keep decreasing and the hydrogen ions increase, pH
decreases.
See lower equation: The indicator equilibrium shifts left, In
-
ions
decrease. Below pH 8.2 the indicator is colorless. As H
+
ions are
further increased and pH decreases to pH 4-5, the indicator
equilibrium is effected and changes to the colorless HIn form.
Equilibrium: HIn --> H
+
+ In
-
colorless red
pH range of indicator
INDICATOR COLOR CHANGE pH RANGE
PHENOLPHTHALEIN COLORLESS to PINK 8.3 10.0
METHYL ORANGE RED to ORANGE 3.1- 4.4
METHYL RED RED to YELLOW 4.4 6.0
BROMOTHYMOL BLUE YELLOW to BLUE 6.0 7.6
LITMUS RED to BLUE 5.0 8.0
What is End Point?
In an acid- base titration, the base solution is gradually
added from a burette into an acidic solution in a titration
flask. When the amount of base added neutralize to the
amount of base added in the flask equivalence point or
End point will reached. The end point of the titration is
shown by color changes of an indicator , previously added
to the acidic solution in the titration flask.
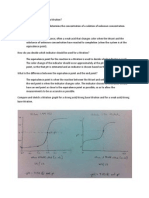
pH CURVE
During an acid base titration, the pH of the solution in the
titration flask changes with the addition of titrant (i.e. base)
from the burette. A plot of pH against the volume of the
solution being added is known as pH curve.
e.g As NaoH is added to Hcl solution the pH of the solution
increases slowly at first, then rapidly in the vicinity of the end
point and again become slow. The end point lies at some place
on this vertical portion.
The colour change would happen when you mix the two
solutions together in exactly equation proportions. That end
point is known as the equivalence point.
Titration curves for strong acid v
strong base
When a strong acid (Hcl) is titrated against a strong base (NaoH),
when NaoH is added the pH increases slowly and gradually upto pH
4. At this pH 4, just 1-2 drops of NaoH from the burrette cause a
sharp change in pH from 4-10, and we get vertical portion of pH
curve which may extend from pH 4 to pH 10. at this vertical portion
there lies the end point. So any indicator which has pH range in this
vertical portion range will be suitable indicator for this type of
titration.
e.g. Phenolphthalein ( 8.3- 10.0)
Methyl red ( 4.4- 6.0)
Titration curves for strong acid v
weak base
When a strong acid (Hcl) is titrated against a weak base (NH4oH), its
pH increases slowly and gradually upto pH 3. At this pH 3, just 1-2
drops of NaoH from the burrette cause a sharp change in pH from 3-
8, and we get vertical portion of pH curve which may extend from pH
3 to pH 8 at this vertical portion there lies the end point. So any
indicator which has pH range in this vertical portion range will be
suitable indicator for this type of titration.
e.g. Methyl orange ( 3.1- 4.4)
Methyl red ( 4.4- 6.0)
It is only after the equivalence point that things become different.
A buffer solution is formed containing excess ammonium chloride.
This resists any large increase in pH - not that you would expect a
very large increase anyway, because ammonia is only a weak base.
Titration curves for weak acid v
Strong base
When a weak acid (CH3COOH) is titrated against a strong base
(NaoH), its pH increases very rapidly and gradually upto pH 6. At this
pH 6, just 1-2 drops of NaoH from the burrette cause a sharp change
in pH from 6-11, and we get vertical portion of pH curve which may
extend from pH 6 to pH 11 at this vertical portion there lies the end
point. So any indicator which has pH range in this vertical portion
range will be suitable indicator for this type of titration.
e.g. phenolphthalein (8.3- 10.00)
The start of the graph shows a relatively rapid rise in pH but this slows down as a
buffer solution is produced. Beyond the equivalence point (when the sodium
hydroxide is in excess) the curve is just the same as that end of the HCl - NaOH
graph.
Titration curves for weak acid v
weakbase
When a weak acid (CH3COOH) is titrated against a weak base
(NH4oH), its pH increases very slowly and there in no sharp change
in pH around end point, so missed a vertical portion of pH curve. So
no suitable indicator for this type of titration.
It so happens that these two are both about equally weak - in that
case, the equivalence point is approximately pH 7.
Notice that there isn't any steep bit on this graph.
Instead, there is just what is known as a "point of
inflexion". That lack of a steep bit means that it is difficult
to do a titration of a weak acid against a weak base
UNIVERSAL INDICATOR
A Universal indicator is a pH indicator composed of a solution of several
compounds that exhibits several smooth colour changes over a pH value range
from 1-14 to indicate the acidity or alkalinity of solutions. universal indicator is
typically composed of water, propan-1-ol, phenolphthalein sodium salt, sodium
hydroxide, methyl red, bromothymol blue monosodium salt, and thymol blue
monosodium salt. The colours that indicate the pH of a solution, after adding a
universal indicator are:
pH range Description Colour
3< Strong acid Red
3-6 Acid Orange/Yellow
7 Neutral Green
8-11 alkali Blue
> 11 Strong alkali Violet/Purple
You might also like
- Collision TheoryDocument24 pagesCollision TheoryKHAIRUNNISALOQMANNo ratings yet
- Plant Water RelationsDocument27 pagesPlant Water RelationsNam GonzalesNo ratings yet
- Affinity Laws For Centrifugal PumpsDocument3 pagesAffinity Laws For Centrifugal PumpshalerNo ratings yet
- Production of polyaluminum chloride (PAC) process flow diagramDocument7 pagesProduction of polyaluminum chloride (PAC) process flow diagramMuhammad Khairul Nizam SuidNo ratings yet
- Preparation of Potassium Sodium TartrateDocument7 pagesPreparation of Potassium Sodium TartrateMina BiancaNo ratings yet
- pH = 4.78So oxalic acid can only be titrated as diprotic acidDocument35 pagespH = 4.78So oxalic acid can only be titrated as diprotic acidŠĭlệncěIšmyPŕIdệNo ratings yet
- Structural IsomerismDocument12 pagesStructural IsomerismAina ReduzanNo ratings yet
- Thermodynamic CyclesDocument14 pagesThermodynamic CyclesBubai111No ratings yet
- SN1 ReactionDocument17 pagesSN1 Reactionsp_putulNo ratings yet
- Experiment 8 Synthesis of An Azo Dye - The Coupling Reaction of Benzenediazonium Ion With Naphthalen-2-OlDocument9 pagesExperiment 8 Synthesis of An Azo Dye - The Coupling Reaction of Benzenediazonium Ion With Naphthalen-2-Olana pertiwiNo ratings yet
- PAC Vs ALUMDocument6 pagesPAC Vs ALUMKristofer WeeksNo ratings yet
- Induction Hardening (Bayu-Bowo)Document22 pagesInduction Hardening (Bayu-Bowo)zaid sulaimanNo ratings yet
- Engineering MaterialsDocument25 pagesEngineering MaterialsNichan CanilloNo ratings yet
- Azeotropic Distillation of Toluene With MethanolDocument16 pagesAzeotropic Distillation of Toluene With MethanolNurtasha AtikahNo ratings yet
- Tutorial SheetsDocument11 pagesTutorial SheetsKAMARAJU SAI VAMSHINo ratings yet
- Hazards of PolymersDocument9 pagesHazards of PolymersTalha Shaukat67% (3)
- Chemical Formula of Copper Chloride HydrateDocument9 pagesChemical Formula of Copper Chloride HydrateAatika SyedNo ratings yet
- PDF Friction - Chapter 3Document17 pagesPDF Friction - Chapter 3Loranie SulukangNo ratings yet
- SCYA2101 Engineering Chemistry Lab Manual Final Copy For WebsiteDocument41 pagesSCYA2101 Engineering Chemistry Lab Manual Final Copy For WebsiteSivaSaiNo ratings yet
- PAC Proses PDFDocument100 pagesPAC Proses PDFAdnan FirdausNo ratings yet
- Titrimetric Methods Precipitation TitrimetryDocument40 pagesTitrimetric Methods Precipitation TitrimetryYasherly AmrinaNo ratings yet
- Mill Scale Safety Data SheetDocument7 pagesMill Scale Safety Data SheetNeni RahayuNo ratings yet
- Water Quality Monitoring SystemDocument2 pagesWater Quality Monitoring Systemvts10573cndps.comNo ratings yet
- CHEM 103 Exp 10 Standardization NaOHDocument3 pagesCHEM 103 Exp 10 Standardization NaOHgiorgyaNo ratings yet
- Self Heating Food PackagingDocument23 pagesSelf Heating Food Packagingankita pathaniaNo ratings yet
- Instrumental Methods of AnalysisDocument10 pagesInstrumental Methods of AnalysisChemistry BNMITNo ratings yet
- Colorimetric Determination of PH FINALDocument32 pagesColorimetric Determination of PH FINALAnn Renette UyNo ratings yet
- Density LabDocument3 pagesDensity Labapi-237572573No ratings yet
- Polyaluminumchlorideproduction PDFDocument7 pagesPolyaluminumchlorideproduction PDFDũng LêNo ratings yet
- Zinc MsdsDocument5 pagesZinc MsdsJohn AtkinsNo ratings yet
- Chemicals Blowing Agent in The Rubber IndustryDocument10 pagesChemicals Blowing Agent in The Rubber IndustryBenpetro ChaichuaNo ratings yet
- PolarimetryDocument1 pagePolarimetryNaimNo ratings yet
- Nucleophile PDFDocument31 pagesNucleophile PDFShrikantSaxenaNo ratings yet
- Ceramic Mug Material AnalysisDocument12 pagesCeramic Mug Material AnalysisLydia HoNo ratings yet
- Test SolutionDocument9 pagesTest Solutionlu_nguyenNo ratings yet
- Speed Control of An Induction Motor Using Raspberry PIDocument7 pagesSpeed Control of An Induction Motor Using Raspberry PIJosé De Jesús PCNo ratings yet
- Drilling Fluid Test Procedure: Filtration TestsDocument5 pagesDrilling Fluid Test Procedure: Filtration TestsInam Ali AwanNo ratings yet
- Redox TitrationDocument27 pagesRedox TitrationthereseNo ratings yet
- Calibration CurveDocument10 pagesCalibration Curvelee diquiatco0% (1)
- PH INDICATORDocument14 pagesPH INDICATORaddan.gull98No ratings yet
- IndicatorsDocument6 pagesIndicatorsRajeev GangwarNo ratings yet
- Theory of Indicators Ostwalds TheoryDocument3 pagesTheory of Indicators Ostwalds TheoryKala SuvarnaNo ratings yet
- Acid-Base TitrationDocument16 pagesAcid-Base TitrationChloe KittyNo ratings yet
- IndicatorsDocument26 pagesIndicatorsDeepa DevanathanNo ratings yet
- Non Aqueous TitrationDocument15 pagesNon Aqueous TitrationDr Priti JainNo ratings yet
- Unit 2 Pharmaceutical AnalysisDocument15 pagesUnit 2 Pharmaceutical AnalysisBharath AthanikarNo ratings yet
- Chemistry Lab 10 Keresa HaughtonDocument5 pagesChemistry Lab 10 Keresa HaughtonKayenNo ratings yet
- AssignmentDocument15 pagesAssignmentIsrat Jahan SurovyNo ratings yet
- Acid Base IndicatorsDocument7 pagesAcid Base IndicatorsAsif Hasan Niloy100% (1)
- Assignment 1 BP102Document15 pagesAssignment 1 BP102Angela BaliNo ratings yet
- Elements of TitrationDocument4 pagesElements of TitrationChyna M. MangibinNo ratings yet
- Assignment: TopicDocument16 pagesAssignment: TopicIsrat Jahan SurovyNo ratings yet
- UNIT II Acid Base TitrationDocument48 pagesUNIT II Acid Base TitrationDr Priti JainNo ratings yet
- Ostwald's Theory of Acid and Base IndicatorsDocument12 pagesOstwald's Theory of Acid and Base IndicatorsPushkar WaneNo ratings yet
- 19 Reactions of Acids and BasesDocument19 pages19 Reactions of Acids and BasesrachelelizabethNo ratings yet
- Theory - of - Indicators OswalDocument3 pagesTheory - of - Indicators OswalHitansh KotadiyaNo ratings yet
- 19.2 Acid-Base Titration CurvesDocument9 pages19.2 Acid-Base Titration CurvesYuyun Sri IriantiNo ratings yet
- QuestionsDocument3 pagesQuestionsapi-203606735No ratings yet
- Acid-Base TitrationsDocument34 pagesAcid-Base TitrationsAisha IltafNo ratings yet
- 1.ACIDS AND BASES Lecture 1Document6 pages1.ACIDS AND BASES Lecture 1Protusha RakshitNo ratings yet
- Hydride MetalDocument29 pagesHydride MetalAdil AminNo ratings yet
- Validated Spectroscopic Method For Estimation of Naproxen From Tablet FormulationDocument3 pagesValidated Spectroscopic Method For Estimation of Naproxen From Tablet FormulationAdil AminNo ratings yet
- TableDocument1 pageTableAdil AminNo ratings yet
- FLIX Booking 8008365935Document3 pagesFLIX Booking 8008365935Adil AminNo ratings yet
- Shear Rate Simulation in CSTR On CFDDocument7 pagesShear Rate Simulation in CSTR On CFDAdil AminNo ratings yet
- Industrial Psychology and SociologyDocument90 pagesIndustrial Psychology and SociologyAdil AminNo ratings yet
- The Theory of Everything - Scientist and Stereotype in Literature and FilmDocument2 pagesThe Theory of Everything - Scientist and Stereotype in Literature and FilmAdil AminNo ratings yet
- Instabilities of droplets in microfluidic Hele-Shaw cellsDocument12 pagesInstabilities of droplets in microfluidic Hele-Shaw cellsAdil AminNo ratings yet
- 15 J 5973Document20 pages15 J 5973Adil AminNo ratings yet
- 2004-04 Nannoolal Masters ThesisDocument202 pages2004-04 Nannoolal Masters ThesisAdil AminNo ratings yet
- A Guidebook To Particle Size AnalysisDocument32 pagesA Guidebook To Particle Size Analysisghandri1986No ratings yet
- High-Performance Liquid ChromatographyDocument5 pagesHigh-Performance Liquid ChromatographyYulia PrimasariNo ratings yet
- Physical and Chemical Properties Analysis of Jatropha Curcas Seed Oil For Industrial ApplicationsDocument4 pagesPhysical and Chemical Properties Analysis of Jatropha Curcas Seed Oil For Industrial ApplicationsAdil AminNo ratings yet
- SolutionDocument89 pagesSolutionAdil AminNo ratings yet
- Optimal Design and Operation of Wastewater Treatment PlantsDocument236 pagesOptimal Design and Operation of Wastewater Treatment PlantsFlorian_AngererNo ratings yet
- CrystallizationDocument40 pagesCrystallizationAdil AminNo ratings yet
- Chapter 2: Heat Exchangers Rules of Thumb For Chemical Engineers, 5th Edition by Stephen HallDocument85 pagesChapter 2: Heat Exchangers Rules of Thumb For Chemical Engineers, 5th Edition by Stephen HallNadirah RahmanNo ratings yet
- Distillation LectureDocument32 pagesDistillation LectureAdil AminNo ratings yet
- CSS Essay Writing Style - I Scored 8 Bands. Keys ExplainedDocument3 pagesCSS Essay Writing Style - I Scored 8 Bands. Keys ExplainedAdil AminNo ratings yet
- BiodieselDocument5 pagesBiodieselAdil AminNo ratings yet
- Article1381242112 - Bilal Et AlDocument8 pagesArticle1381242112 - Bilal Et AlAdil AminNo ratings yet
- AduDocument8 pagesAduAdil AminNo ratings yet
- Lyophilization/Freeze Drying: Dr. Nasir AbbasDocument12 pagesLyophilization/Freeze Drying: Dr. Nasir AbbasAdil AminNo ratings yet
- Article1381242112 - Bilal Et AlDocument8 pagesArticle1381242112 - Bilal Et AlAdil AminNo ratings yet
- IELTS Tips EnglishDocument16 pagesIELTS Tips Englishkashifrazamangi100% (2)
- 21Document8 pages21Adil AminNo ratings yet
- AduDocument8 pagesAduAdil AminNo ratings yet
- RandomDocument1 pageRandomAdil AminNo ratings yet
- Chapter 1Document61 pagesChapter 1SyahshaiNo ratings yet
- Eam of Analysis EditedDocument140 pagesEam of Analysis EditedAvishek KumarNo ratings yet
- VOLUMETRIC AnalysisDocument49 pagesVOLUMETRIC AnalysisLisa Dea SaryNo ratings yet
- Topic:-Analysis of Purity of DrugsDocument44 pagesTopic:-Analysis of Purity of DrugsAditya SharmaNo ratings yet
- Membranes International CationDocument1 pageMembranes International CationjfdezmtnezNo ratings yet
- Ch17 TestbankDocument38 pagesCh17 TestbankJeremy Martin80% (5)
- Lowry AssayDocument7 pagesLowry AssayGrace AquinoNo ratings yet
- Quantitative Determination of Total Hardness in Drinking Water by Complexometric Edta TitrationDocument4 pagesQuantitative Determination of Total Hardness in Drinking Water by Complexometric Edta TitrationEXO SVTNo ratings yet
- Aspirin TitrationDocument3 pagesAspirin TitrationBiancaTardecillaNo ratings yet
- Ultracentrifuge - Wikipedia, The Free EncyclopediaDocument3 pagesUltracentrifuge - Wikipedia, The Free EncyclopediaShailendra YadavNo ratings yet
- Gas Absorption in Packed Tower (S1 2015) (Note)Document51 pagesGas Absorption in Packed Tower (S1 2015) (Note)venkieeNo ratings yet
- Kesetimbangan LarutanDocument123 pagesKesetimbangan LarutanFirda SafitriNo ratings yet
- Practical Quantitative Biomedical Applications of MALDI-TOF Mass SpectrometryDocument13 pagesPractical Quantitative Biomedical Applications of MALDI-TOF Mass SpectrometryDiana ReyNo ratings yet
- Batch Distillation: Group 7 Errynne Yanza Hosleck Galasinao Aron BalinesDocument18 pagesBatch Distillation: Group 7 Errynne Yanza Hosleck Galasinao Aron BalinesAron BalinesNo ratings yet
- Research ArticleDocument9 pagesResearch Articleade muchlasNo ratings yet
- European Pharmacopoeia Fusidic Acid TestsDocument1 pageEuropean Pharmacopoeia Fusidic Acid Testsnarutotriyan9350No ratings yet
- IP 3. Protocol - Chemical Principles II LaboratoryDocument9 pagesIP 3. Protocol - Chemical Principles II LaboratoryJavier PratdesabaNo ratings yet
- Atomic Absorption SpectrosDocument9 pagesAtomic Absorption Spectrosamirul azhar80% (10)
- Determine Acid Content of Fruit Juices (40Document8 pagesDetermine Acid Content of Fruit Juices (40jules blancoNo ratings yet
- ChemCAD Presentation Explains Chemical Process SimulationDocument24 pagesChemCAD Presentation Explains Chemical Process SimulationJohn Unk100% (1)
- Experiment 8 - Complexometric TitrationDocument7 pagesExperiment 8 - Complexometric TitrationJoemer Absalon Adorna100% (1)
- Test Bank For Organic Chemistry 4 Edition Janice SmithDocument24 pagesTest Bank For Organic Chemistry 4 Edition Janice SmithStevenMcclainpwdt100% (47)
- Analytical Chemistry (CHM111) Laboratory ManualDocument73 pagesAnalytical Chemistry (CHM111) Laboratory ManualKatrina BucudNo ratings yet
- Introduction TitrationDocument30 pagesIntroduction TitrationlacaranjaredNo ratings yet
- Analysis of Peppermint Leaf and Spearmint Leaf ExtractsDocument4 pagesAnalysis of Peppermint Leaf and Spearmint Leaf ExtractsJonathan MaresNo ratings yet
- Protein purification pre-class exerciseDocument4 pagesProtein purification pre-class exerciseKathleen GomezNo ratings yet
- ANJAC Science Instrumentation Centre ChargesDocument4 pagesANJAC Science Instrumentation Centre Charges16 Malola KrishnanNo ratings yet
- Physical Pharmacy 3 PDFDocument10 pagesPhysical Pharmacy 3 PDFhusseinNo ratings yet
- TLC PDFDocument9 pagesTLC PDFMilica RančićNo ratings yet
- Calculating MolarityDocument3 pagesCalculating Molarityapi-253461549No ratings yet