Professional Documents
Culture Documents
Benzena Dan Aromatisan
Uploaded by
Shelly Trissa Ramadhan0 ratings0% found this document useful (0 votes)
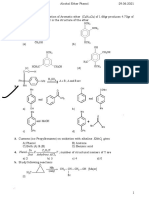
61 views34 pagesThe document discusses benzene and aromatic compounds. It defines benzene as the simplest aromatic hydrocarbon and describes its structure as a six-membered ring with alternating single and double bonds. While benzene reacts differently than other unsaturated hydrocarbons, undergoing substitution rather than addition reactions. The key aspects of aromaticity are outlined, including being cyclic, planar, fully conjugated, and satisfying Hückel's rule of having 4n+2 pi electrons.
Original Description:
Original Title
Benzena dan Aromatisan.ppt
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses benzene and aromatic compounds. It defines benzene as the simplest aromatic hydrocarbon and describes its structure as a six-membered ring with alternating single and double bonds. While benzene reacts differently than other unsaturated hydrocarbons, undergoing substitution rather than addition reactions. The key aspects of aromaticity are outlined, including being cyclic, planar, fully conjugated, and satisfying Hückel's rule of having 4n+2 pi electrons.
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
61 views34 pagesBenzena Dan Aromatisan
Uploaded by
Shelly Trissa RamadhanThe document discusses benzene and aromatic compounds. It defines benzene as the simplest aromatic hydrocarbon and describes its structure as a six-membered ring with alternating single and double bonds. While benzene reacts differently than other unsaturated hydrocarbons, undergoing substitution rather than addition reactions. The key aspects of aromaticity are outlined, including being cyclic, planar, fully conjugated, and satisfying Hückel's rule of having 4n+2 pi electrons.
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 34
Benzene and Aromatic Compounds
Background
Benzene (C6H6) is the simplest aromatic hydrocarbon (or
arene).
Benzene has four degrees of unsaturation, making it a
highly unsaturated hydrocarbon.
Whereas unsaturated hydrocarbons such as alkenes,
alkynes and dienes readily undergo addition reactions,
benzene does not.
Benzene and Aromatic Compounds
Background
Benzene does react with bromine, but only in the presence of
FeBr3 (a Lewis acid), and the reaction is a substitution, not an
addition.
Proposed structures of benzene must account for its high degree
of unsaturation and its lack of reactivity towards electrophilic
addition.
August Kekul proposed that benzene was a rapidly
equilibrating mixture of two compounds, each containing a sixmembered ring with three alternating bonds.
In the Kekul description, the bond between any two carbon
atoms is sometimes a single bond and sometimes a double
bond.
2
Benzene and Aromatic Compounds
Background
These structures are known as Kekul structures.
Although benzene is still drawn as a six-membered ring with
alternating bonds, in reality there is no equilibrium between the
two different kinds of benzene molecules.
Current descriptions of benzene are based on resonance and
electron delocalization due to orbital overlap.
In the nineteenth century, many other compounds having
properties similar to those of benzene were isolated from natural
sources. Since these compounds possessed strong and
characteristic odors, they were called aromatic compounds. It
should be noted, however, that it is their chemical properties,
and not their odor, that make them special.
Benzene and Aromatic Compounds
The Structure of Benzene
Any structure for benzene must account for the following facts:
1. It contains a six-membered ring and three additional
degrees of unsaturation.
2. It is planar.
3. All CC bond lengths are equal.
The Kekul structures satisfy the first two criteria but not the
third, because having three alternating bonds means that
benzene should have three short double bonds alternating with
three longer single bonds.
Benzene and Aromatic Compounds
The Structure of Benzene
The resonance description of benzene consists of two equivalent
Lewis structures, each with three double bonds that alternate
with three single bonds.
The true structure of benzene is a resonance hybrid of the two
Lewis structures, with the dashed lines of the hybrid indicating
the position of the bonds.
We will use one of the two Lewis structures and not the hybrid in
drawing benzene. This will make it easier to keep track of the
electron pairs in the bonds (the electrons).
Benzene and Aromatic Compounds
The Structure of Benzene
Because each bond has two electrons, benzene has
six electrons.
Benzene and Aromatic Compounds
The Structure of Benzene
In benzene, the actual bond length (1.39 ) is
intermediate between the carboncarbon single bond
(1.53 ) and the carboncarbon double bond (1.34 ).
Benzene and Aromatic Compounds
Nomenclature of Benzene Derivatives
To name a benzene ring with one substituent, name the
substituent and add the word benzene.
Many monosubstituted benzenes have common names
which you must also learn.
Benzene and Aromatic Compounds
Nomenclature of Benzene Derivatives
There are three different ways that two groups can be
attached to a benzene ring, so a prefixortho, meta, or
paracan be used to designate the relative position of
the two substituents.
ortho-dibromobenzene
or
o-dibromobenzene
or 1,2-dibromobenzene
meta-dibromobenzene
or
m-dibromobenzene
or 1,3-dibromobenzene
para-dibromobenzene
or
p-dibromobenzene
or 1,4-dibromobenzene
Benzene and Aromatic Compounds
Nomenclature of Benzene Derivatives
If the two groups on the benzene ring are different,
alphabetize the names of the substituents preceding the
word benzene.
If one substituent is part of a common root, name the
molecule as a derivative of that monosubstituted
benzene.
10
Benzene and Aromatic Compounds
Nomenclature of Benzene Derivatives
For three or more substituents on a benzene ring:
1. Number to give the lowest possible numbers around the ring.
2. Alphabetize the substituent names.
3. When substituents are part of common roots, name the
molecule as a derivative of that monosubstituted benzene. The
substituent that comprises the common root is located at C1.
11
Benzene and Aromatic Compounds
Nomenclature of Benzene Derivatives
A benzene substituent is called a phenyl group, and it can be
abbreviated in a structure as Ph-.
Therefore, benzene can be represented as PhH, and phenol
would be PhOH.
12
Benzene and Aromatic Compounds
Nomenclature of Benzene Derivatives
The benzyl group, another common substituent that contains a
benzene ring, differs from a phenyl group.
Substituents derived from other substituted aromatic rings are
collectively known as aryl groups.
13
Benzene and Aromatic Compounds
Interesting Aromatic Compounds
Benzene and toluene, the simplest aromatic hydrocarbons
obtained from petroleum refining, are useful starting materials
for synthetic polymers. They are also two of the components of
the BTX mixture added to gasoline to boost octane ratings.
Compounds containing two or more benzene rings that share
carboncarbon bonds are called polycyclic aromatic
hydrocarbons (PAHs). Naphthalene, the simplest PAH, is the
active ingredient in mothballs.
14
Benzene and Aromatic Compounds
Interesting Aromatic Compounds
Figure 17.3
Benzo[a]pyrene, a
common PAH
When ingested or inhaled, benzo[a]pyrene and other similar
PAHs are oxidized to carcinogenic products.
15
Benzene and Aromatic Compounds
Interesting Aromatic Compounds
Figure 17.5
Selected drugs that contain
a benzene ring
16
Benzene and Aromatic Compounds
Stability of Benzene
Consider the heats of hydrogenation of cyclohexene, 1,3cyclohexadiene and benzene, all of which give cyclohexane
when treated with excess hydrogen in the presence of a metal
catalyst.
17
Benzene and Aromatic Compounds
Stability of Benzene
Figure 17.6 compares the hypothetical and observed heats of
hydrogenation for benzene.
Figure 17.6
A comparison between the
observed and hypothetical
heats of hydrogenation
for benzene
The huge difference between the hypothetical and observed heats
of hydrogenation for benzene cannot be explained solely on the
18
basis of resonance and conjugation.
Benzene and Aromatic Compounds
Stability of Benzene
The low heat of hydrogenation of benzene means that benzene is
especially stableeven more so than conjugated polyenes. This
unusual stability is characteristic of aromatic compounds.
Benzenes unusual behavior is not limited to hydrogenation.
Benzene does not undergo addition reactions typical of other
highly unsaturated compounds, including conjugated dienes.
Benzene does not react with Br2 to yield an addition product.
Instead, in the presence of a Lewis acid, bromine substitutes for
a hydrogen atom, yielding a product that retains the benzene
ring.
19
Benzene and Aromatic Compounds
The Criteria for AromaticityHckels Rule
Four structural criteria must be satisfied for a compound
to be aromatic.
[1] A molecule must be cyclic.
To be aromatic, each p orbital must overlap with p orbitals
on adjacent atoms.
20
Benzene and Aromatic Compounds
The Criteria for AromaticityHckels Rule
[2] A molecule must be planar.
All adjacent p orbitals must be aligned so that the
electron density can be delocalized.
Since cyclooctatetraene is non-planar, it is not aromatic,
and it undergoes addition reactions just like those of other
alkenes.
21
Benzene and Aromatic Compounds
The Criteria for AromaticityHckels Rule
[3] A molecule must be completely conjugated.
Aromatic compounds must have a p orbital on every atom.
22
Benzene and Aromatic Compounds
The Criteria for AromaticityHckels Rule
[4] A molecule must satisfy Hckels rule, and contain
a particular number of electrons.
Hckel's rule:
Benzene is aromatic and especially stable because it
contains 6 electrons. Cyclobutadiene is antiaromatic
and especially unstable because it contains 4 electrons.
23
Benzene and Aromatic Compounds
The Criteria for AromaticityHckels Rule
Note that Hckels rule refers to the number of
electrons, not the number of atoms in a particular ring.
24
Benzene and Aromatic Compounds
The Criteria for AromaticityHckels Rule
Considering aromaticity, a compound can be classified in
one of three ways:
1. AromaticA cyclic, planar, completely conjugated
compound with 4n + 2 electrons.
2. AntiaromaticA cyclic, planar, completely conjugated
compound with 4n electrons.
3. Not aromatic (nonaromatic)A compound that lacks
one (or more) of the following requirements for
aromaticity: being cyclic, planar, and completely
conjugated.
25
Benzene and Aromatic Compounds
The Criteria for AromaticityHckels Rule
Note the relationship between each compound type and a similar
open-chained molecule having the same number of electrons.
26
Benzene and Aromatic Compounds
The Criteria for AromaticityHckels Rule
The protons on sp2 hybridized carbons in aromatic
hydrocarbons are highly deshielded and absorb at 6.5-8
ppm, whereas hydrocarbons that are not aromatic
absorb at 4.5-6 ppm.
27
Benzene and Aromatic Compounds
Examples of Aromatic Rings
Completely conjugated rings larger than benzene are
also aromatic if they are planar and have 4n + 2
electrons.
Hydrocarbons containing a single ring with alternating
double and single bonds are called annulenes.
To name an annulene, indicate the number of atoms in
the ring in brackets and add the word annulene.
28
Benzene and Aromatic Compounds
Examples of Aromatic Rings
[10]-Annulene has 10 electrons, which satisfies
Hckel's rule, but a planar molecule would place the two
H atoms inside the ring too close to each other. Thus,
the ring puckers to relieve this strain.
Since [10]-annulene is not planar, the 10 electrons
cant delocalize over the entire ring and it is not
aromatic.
29
Benzene and Aromatic Compounds
Examples of Aromatic Rings
Two or more six-membered rings with alternating double and
single bonds can be fused together to form polycyclic aromatic
hydrocarbons (PAHs).
There are two different ways to join three rings together, forming
anthracene and phenanthrene.
As the number of fused rings increases, the number of
resonance structures increases. Naphthalene is a hybrid of three
resonance structures whereas benzene is a hybrid of two.
30
Benzene and Aromatic Compounds
Examples of Aromatic Rings
Heterocycles containing oxygen, nitrogen or sulfur, can also be
aromatic.
With heteroatoms, we must determine whether the lone pair is
localized on the heteroatom or part of the delocalized system.
An example of an aromatic heterocycle is pyridine.
31
Benzene and Aromatic Compounds
Examples of Aromatic Rings
Pyrrole is another example of an aromatic heterocycle. It
contains a five-membered ring with two bonds and one
nitrogen atom.
Pyrrole has a p orbital on every adjacent atom, so it is
completely conjugated.
Pyrrole has six electronsfour from the bonds and two from
the lone pair.
Pyrrole is cyclic, planar, completely conjugated, and has 4n + 2
32
electrons, so it is aromatic.
Benzene and Aromatic Compounds
Examples of Aromatic Rings
Histamine is a biologically active amine formed in many tissues.
It is an aromatic heterocycle with two N atomsone which is
similar to the N atom of pyridine, and the other which is similar
to the N atom of pyrrole.
33
Benzene and Aromatic Compounds
Examples of Aromatic Rings
Both negatively and positively charged ions can be aromatic if
they possess all the necessary elements.
We can draw five equivalent resonance structures for the
34
cyclopentadienyl anion.
You might also like
- Benzene MilanaDocument61 pagesBenzene MilanaMilana WalujoNo ratings yet
- Organic Chemistry,: Benzene & AromaticsDocument41 pagesOrganic Chemistry,: Benzene & AromaticsRIZKI ALDINO AHMAD 1506723912No ratings yet
- Aromatic CompoundsDocument56 pagesAromatic CompoundsSeth Andrew Salih100% (2)
- Chapter 17 - BENZENE AND AROMATICSDocument28 pagesChapter 17 - BENZENE AND AROMATICSsalman alfarizziNo ratings yet
- 4 Benzene PDFDocument90 pages4 Benzene PDFCrishen VinzonNo ratings yet
- CY 1001: Organic Chemistry: Aromaticity: (4 Classes + 1 Tutorial)Document80 pagesCY 1001: Organic Chemistry: Aromaticity: (4 Classes + 1 Tutorial)Sai naveenNo ratings yet
- Chapter 2.5 Aromatic CompoundDocument38 pagesChapter 2.5 Aromatic Compound0JTINGNo ratings yet
- Aromatic HydrocarbonsDocument50 pagesAromatic HydrocarbonsEdan Balao-asNo ratings yet
- Benzene and Aromaticity (2nd Year Ndaweni)Document54 pagesBenzene and Aromaticity (2nd Year Ndaweni)Mbali MazongweNo ratings yet
- BenzeneDocument21 pagesBenzeneosamakhan8967No ratings yet
- Edited 2022 - Aromatic CompoundsDocument73 pagesEdited 2022 - Aromatic CompoundsedinapetermugaduiNo ratings yet
- 307 Aromatic CompoundDocument42 pages307 Aromatic CompoundFAHEEM UD DINNo ratings yet
- Dienes & Aromatic Compounds, FNDocument60 pagesDienes & Aromatic Compounds, FNMuzahidul IslamNo ratings yet
- Materi 03Document38 pagesMateri 03Syukri DaimonNo ratings yet
- Aromatic CompoundsDocument30 pagesAromatic CompoundsMA Masum HossainNo ratings yet
- Chapter 6 Aromatic CompoundsDocument41 pagesChapter 6 Aromatic Compoundsnur izzaidahNo ratings yet
- Aromatic CompoundsDocument36 pagesAromatic CompoundsMaria Ruela Agodera SumogNo ratings yet
- Corrected Fundamentals of Organic ChemistryDocument71 pagesCorrected Fundamentals of Organic ChemistryDAM2120No ratings yet
- 1 BenzeneDocument41 pages1 Benzeneraj royelNo ratings yet
- Organic Chemistry II / CHEM 252 Chapter 14 - : Aromatic CompoundsDocument28 pagesOrganic Chemistry II / CHEM 252 Chapter 14 - : Aromatic CompoundsLurthu PushparajNo ratings yet
- Benzene Aromatic CompoundsDocument14 pagesBenzene Aromatic CompoundsBook of Life fgfhfghfghfghNo ratings yet
- C2 Benzene & AromaticityDocument74 pagesC2 Benzene & AromaticityMimi Sharina HassanNo ratings yet
- Aromatic Hydrocarbons Pharmaceutical Organic Chemistry Porg111Document9 pagesAromatic Hydrocarbons Pharmaceutical Organic Chemistry Porg111AnnaGueseNo ratings yet
- Benzene: Milton Biswas Student Madhav University, Abu RoadDocument23 pagesBenzene: Milton Biswas Student Madhav University, Abu RoadMilton BiswasNo ratings yet
- Organic Chemistry - Chapter 15 Benzene & Aromatic CompoundsDocument9 pagesOrganic Chemistry - Chapter 15 Benzene & Aromatic CompoundsSairille ManejaNo ratings yet
- Aromatic Compounds: © 2006 Thomson Higher EducationDocument103 pagesAromatic Compounds: © 2006 Thomson Higher Educationbrigittanwp putriNo ratings yet
- Aromatic Hydrocarbons Unit For SuccessDocument54 pagesAromatic Hydrocarbons Unit For SuccessN210084 CHOULA MANIKANTANo ratings yet
- WEEK 10 Aromatic HydrocarbonDocument26 pagesWEEK 10 Aromatic HydrocarbonChris Angelo De GuzmanNo ratings yet
- OCR Chemistry NotesDocument10 pagesOCR Chemistry NotesJack WoodNo ratings yet
- Benzene and Aromaticity: Based On Mcmurry'S Organic Chemistry, 9 EditionDocument68 pagesBenzene and Aromaticity: Based On Mcmurry'S Organic Chemistry, 9 Edition張湧浩No ratings yet
- STK 1233 Organic Chemistry 1: LU 5.1: Aromatic CompoundsDocument37 pagesSTK 1233 Organic Chemistry 1: LU 5.1: Aromatic CompoundsArllen Joy AlbertNo ratings yet
- Pharmaceutical Organic Chemistry II NotesDocument59 pagesPharmaceutical Organic Chemistry II NotesSayyeda SumaiyahNo ratings yet
- Chapter 4 Aromatic HydrocarbonsDocument34 pagesChapter 4 Aromatic HydrocarbonsAbdirashid Adam IsakNo ratings yet
- Why This Chapter?: Aromatic CompoundsDocument8 pagesWhy This Chapter?: Aromatic CompoundsbebsybiswezNo ratings yet
- BenzeneDocument14 pagesBenzeneJueeli More100% (1)
- Aromatic Hydrocarbons (Benzene) : Name of Aromatic CompoundsDocument23 pagesAromatic Hydrocarbons (Benzene) : Name of Aromatic CompoundsTarunesh PandeyNo ratings yet
- Aromatic HydrocarbonDocument45 pagesAromatic HydrocarbonPrashantNo ratings yet
- 7 Benzene and AromaticsDocument72 pages7 Benzene and Aromaticshamdy solimanNo ratings yet
- NEPHAR 109 Chapter14Document24 pagesNEPHAR 109 Chapter14Amirabbas SaffariNo ratings yet
- Benzene and Its Derivatives: Key QuestionsDocument58 pagesBenzene and Its Derivatives: Key QuestionsBrian GichanaNo ratings yet
- Organic Chemistry For USTH Students Benzene and Aromatic SystemsDocument69 pagesOrganic Chemistry For USTH Students Benzene and Aromatic SystemsHoàng Hiệp100% (1)
- Aromatic It yDocument9 pagesAromatic It yBentenBentenNo ratings yet
- Aromaticity CompleteDocument104 pagesAromaticity Completewahidalwahdi100% (1)
- Chem 242-Chapter Lecture 1-1Document46 pagesChem 242-Chapter Lecture 1-1Hossam El-basiounyNo ratings yet
- Aromatic Hydrocarbons - 1620192910Document40 pagesAromatic Hydrocarbons - 1620192910Sahisa MahatNo ratings yet
- Organic Chemistry Module 4Document5 pagesOrganic Chemistry Module 4Josephine TeroNo ratings yet
- C H H H H H: Hydrocarbons. Methane. AlkanesDocument14 pagesC H H H H H: Hydrocarbons. Methane. AlkanesDavidDavidovNo ratings yet
- Chapter 5 AromaticDocument76 pagesChapter 5 AromaticMELVINDO JACOBNo ratings yet
- Aromatic HydrocarbonDocument31 pagesAromatic HydrocarbonCamille Solana100% (1)
- Aromatic CompoundsDocument55 pagesAromatic CompoundsNadine Bacalangco100% (1)
- LEC 19 Nomen-Culture and Structure of BenzeneDocument16 pagesLEC 19 Nomen-Culture and Structure of BenzeneIftikhar Ud DinNo ratings yet
- B.Sc. Aromatic HydrocarbonsDocument21 pagesB.Sc. Aromatic HydrocarbonsJhoanna MarigmenNo ratings yet
- Aromatic, Benzenoid and Heterocyclic ChemistryDocument24 pagesAromatic, Benzenoid and Heterocyclic Chemistryrosemoses305No ratings yet
- Aromatic HydrocarbonsDocument2 pagesAromatic HydrocarbonsHalloNo ratings yet
- Lesson 4 Aromatic HCDocument24 pagesLesson 4 Aromatic HCEricka LaluzNo ratings yet
- Pharmaceutical Organic Chemistry LecDocument75 pagesPharmaceutical Organic Chemistry Lecبن آجروم50% (2)
- DownloadDocument11 pagesDownloadAspirinNo ratings yet
- Geometrical Isomerism: It is known that a carbon-carbon double bond is made up of one σ bond and one π-bond. The π-bond presents free rotation about the double bond.ThisDocument3 pagesGeometrical Isomerism: It is known that a carbon-carbon double bond is made up of one σ bond and one π-bond. The π-bond presents free rotation about the double bond.ThisKisha KhuranaNo ratings yet
- Concerning Amines: Their Properties, Preparation and ReactionsFrom EverandConcerning Amines: Their Properties, Preparation and ReactionsRating: 2.5 out of 5 stars2.5/5 (2)
- Lampiran: ρ W V W ρ xV W g cm x 20 cmDocument2 pagesLampiran: ρ W V W ρ xV W g cm x 20 cmShelly Trissa RamadhanNo ratings yet
- Korean Quotes I Got This From Someone Website. Thanks To Him I Forget His Website NameDocument3 pagesKorean Quotes I Got This From Someone Website. Thanks To Him I Forget His Website NameShelly Trissa RamadhanNo ratings yet
- Material Safety Data Sheet: 1. Identification of The Substance/Preparation and The Company/UndertakingDocument3 pagesMaterial Safety Data Sheet: 1. Identification of The Substance/Preparation and The Company/UndertakingShelly Trissa RamadhanNo ratings yet
- MSDS H2so4Document7 pagesMSDS H2so4Shelly Trissa RamadhanNo ratings yet
- Premium (MSDS)Document13 pagesPremium (MSDS)Shelly Trissa RamadhanNo ratings yet
- ρ massa gram mL: 1. Menghitung massa jenis a. Massa jenis airDocument6 pagesρ massa gram mL: 1. Menghitung massa jenis a. Massa jenis airShelly Trissa RamadhanNo ratings yet
- Full Download Test Bank For Organic Chemistry 1st Edition by David R Klein PDF Full ChapterDocument34 pagesFull Download Test Bank For Organic Chemistry 1st Edition by David R Klein PDF Full Chapterprivitywoolhall.8hvcd100% (17)
- ORGHEM LAB HydrocarbonsDocument11 pagesORGHEM LAB HydrocarbonsJasmine CatanaNo ratings yet
- bài tập phổ nrmDocument102 pagesbài tập phổ nrmĐức NamNo ratings yet
- Silabo - 17201Document7 pagesSilabo - 17201Enrique Rojas BermudoNo ratings yet
- Wurtz ReactionDocument2 pagesWurtz ReactionAnush KhillareNo ratings yet
- Organic Chem NotesDocument49 pagesOrganic Chem NotesPriyaNo ratings yet
- Notes HydrocarbonsDocument9 pagesNotes HydrocarbonsShiina MashiroNo ratings yet
- Carboxylic Acid and Its Derivatives - ChemistryDocument145 pagesCarboxylic Acid and Its Derivatives - ChemistryYoshitha Kuntumalla100% (1)
- Reaction Reactants Products Conditions Mechanism Other: AlkanesDocument3 pagesReaction Reactants Products Conditions Mechanism Other: AlkanesInzamam A HaqueNo ratings yet
- IUPAC Nomenclature of Organic CompoundsDocument15 pagesIUPAC Nomenclature of Organic Compoundsapi-1986055092% (24)
- Tablas de Nomenclatura Con S N PDocument13 pagesTablas de Nomenclatura Con S N PLucero RamirezNo ratings yet
- Module4 AromaticsDocument6 pagesModule4 AromaticsClaire Jean PasiaNo ratings yet
- Incom. Sr. 29.06.2021 AssignmentDocument5 pagesIncom. Sr. 29.06.2021 AssignmentSrikar SatyaNo ratings yet
- Appendix Kimia FisikaDocument41 pagesAppendix Kimia FisikaPieter SchmidtNo ratings yet
- (L6) Carbon and Its Compounds Class10 PDFDocument19 pages(L6) Carbon and Its Compounds Class10 PDFRekha MishraNo ratings yet
- SLG Chem3 LG 3.5 Reactions of Aldehydes Ketones Nucleophilic Addition With HCNDocument5 pagesSLG Chem3 LG 3.5 Reactions of Aldehydes Ketones Nucleophilic Addition With HCNYo MamaNo ratings yet
- IUPAC Nomenclature - Notes - Yakeen 2.0 2024 (Legend)Document3 pagesIUPAC Nomenclature - Notes - Yakeen 2.0 2024 (Legend)ajmeena834No ratings yet
- Isosteres: 1. Univalent AtomsDocument2 pagesIsosteres: 1. Univalent AtomsjfsantamariameNo ratings yet
- Carboxylic Acids and Their Derivatives.Document31 pagesCarboxylic Acids and Their Derivatives.AmanyNo ratings yet
- Class 10 Chapter 8 Part IDocument52 pagesClass 10 Chapter 8 Part IganeshNo ratings yet
- Lec 5 Aldehyde Ketone Nucleophilic Addition PDFDocument78 pagesLec 5 Aldehyde Ketone Nucleophilic Addition PDFAssyakurNo ratings yet
- Etil Asetat Kelas C Kel 4Document3 pagesEtil Asetat Kelas C Kel 4azizasafira farhanNo ratings yet
- Hydrocarbons UsesDocument3 pagesHydrocarbons UsesAj Benito MalidomNo ratings yet
- Experiment 2Document5 pagesExperiment 2Noor Aini JaafarNo ratings yet
- BioMolecules in One ShotDocument46 pagesBioMolecules in One ShotKESHAV KUMARNo ratings yet
- Tipical Solvent For Cementing ThermoplasticsDocument1 pageTipical Solvent For Cementing ThermoplasticsDamiano CrestanNo ratings yet
- Amines NEET QuestionDocument13 pagesAmines NEET Questionmahi.nitukNo ratings yet
- Organic Chemistry - Module 2Document25 pagesOrganic Chemistry - Module 2ChaithraMalluNo ratings yet
- MC HoDocument4 pagesMC HoMarcelo MagalhãesNo ratings yet
- CA Lesson 5 Aromatic HydrocarbonsDocument16 pagesCA Lesson 5 Aromatic HydrocarbonsAlbaraaAliNo ratings yet