Professional Documents
Culture Documents
Chem 31.1 Experiment 4: Distillation
Uploaded by
rjmaotCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chem 31.1 Experiment 4: Distillation
Uploaded by
rjmaotCopyright:
Available Formats
DISTILLATION
A laboratory technique used in separation
and/or purification of components in a
mixture
The process is mainly based upon the
boiling point of liquid substances
heating vaporizing condensing
Experiment IV: Distillation
DISTILLATION
liquid liquid
mixtures
homogeneous heterogeneous
Experiment IV: Distillation
RAOULT’S LAW
for ideal mixtures
It relates the vapor pressure of components
to the composition of the solution
It assumes ideal behavior, that is, the
physical properties of the components
are identical
Experiment IV: Distillation
RAOULT’S LAW
If the two components are very similar, or in
the limiting case, differ only in isotopic content,
then the vapor pressure of each component
will be equal to the vapor pressure of the pure
substance Po times the mole fraction in the
solution
Experiment IV: Distillation
RAOULT’S LAW
The total vapor pressure Ptot above the solution
is equal to the sum of the vapor pressures of
the two [liquid] components, PA and PB
Experiment IV: Distillation
RAOULT’S LAW
Experiment IV: Distillation
RAOULT’S LAW
Experiment IV: Distillation
RAOULT’S LAW
Experiment IV: Distillation
RAOULT’S LAW
Vapor Pressure
It is the pressure exerted by a vapor in
equilibrium with its non-vapor phases
Boiling Point
The temperature at which the vapor pressure
equals the atmospheric pressure
Vapor Pressure 1/α Boiling Point
Experiment IV: Distillation
AZEOTROPE MIXTURE
A mixture of liquids that has a constant boiling
point because the vapour has the same
composition as the liquid mixture
The components of the solution cannot be
separated by simple distillation
Experiment IV: Distillation
AZEOTROPE MIXTURE
POSITIVE AZEOTROPE
Minimum Temperature
Maximum Pressure
NEGATIVE AZEOTROPE
Maximum Temperature
Minimum Pressure
Experiment IV: Distillation
POSITIVE AZEOTROPE MIXTURE
Experiment IV: Distillation
NEGATIVE AZEOTROPE MIXTURE
Experiment IV: Distillation
KINDS OF DISTILLATION
for homogeneous mixtures
Simple Distillation
Fractional Distillation
for heterogeneous mixtures
Steam Distillation
Experiment IV: Distillation
AZEOTROPE MIXTURE
Ethanol Water
95.5% 4.5%
78.1°C
78.3°C 100°C
positive azeotrope mixture
Experiment IV: Distillation
SIMPLE DISTILLATION
It is usually used only to separate liquids
whose boiling points differ greatly (>70°C) or to
separate liquids from involatile solids or oils. In
the process, all the hot vapors produced are
immediately channelled into a condenser
which cools and condenses the vapors
Therefore, the distillate will not be as pure
Experiment IV: Distillation
SIMPLE DISTILLATION
A simple distillation set-up consists of a boiling
flask (round-bottom flask) attached to an
adapter holding a thermometer (to determine
the boiling temperature of the liquid). The
adapter connects to a condenser into which
cold water is constantly passed through. The
condenser leads into a collection flask for the
purified liquid.
Experiment IV: Distillation
SIMPLE DISTILLATION
Experiment IV: Distillation
SIMPLE DISTILLATION
Experiment IV: Distillation
FRACTIONAL DISTILLATION
It is usually employed with separation of
complex mixtures at small boiling points
difference (about 25°C). It can separate the
mixture into its component parts or fractions
Experiment IV: Distillation
FRACTIONAL DISTILLATION
It is essentially the same as simple distillation except
that a fractionating column is placed between the
boiling flask and the condenser. The glass beads
found in the fractionating column provide "theoretical
plates" on which the refluxing liquid can condense, re-
evaporate, and condense again, essentially distilling
the compound over and over. The more volatile
liquids will tend to push towards the top of the
fractionating column, while less volatile liquid will stay
towards the bottom, giving a better separation
between the liquids
Experiment IV: Distillation
FRACTIONAL DISTILLATION
Experiment IV: Distillation
FRACTIONAL DISTILLATION
Experiment IV: Distillation
SIMPLE vs FRACTIONAL DISTILLATION
Simple distillation Fractional distillation
Advantages • simpler setup than fractional •much better separation between
• faster distillation times liquids than simple distillation
• consumes less energy than •can more readily purify complex
fractional distillation mixtures than simple distillation
Disadvantages • requires the liquids to have • more complicated setup than
large boiling point differences simple distillation
(>70oC) • takes longer for liquids to distil
• gives poorer separation than • consumes more energy than
fractional distillation simple distillation
• only works well with relatively
pure liquids
Best used for: separating relatively pure liquids separating complex mixtures of
with large boiling differences or liquids with smaller boiling point
liquids with solid impurities separations.
Experiment IV: Distillation
SIMPLE vs FRACTIONAL DISTILLATION
Experiment IV: Distillation
STEAM DISTILLATION
It is the process of purifying a substance through
application of steam. It deals with compounds that are
heat sensitive (e.g. natural aromatic compounds).
Steam distillation works on the principle that
immiscible substance when mixed together can lower
the boiling point of each other.
Experiment IV: Distillation
STEAM DISTILLATION
0.864g/mL Xylene Water 0.988g/mL
60% 40%
94.5°C
139.1°C 100°C
positive azeotrope mixture
Experiment IV: Distillation
STEAM DISTILLATION
Many organic compounds tend to decompose at high
sustained temperatures. Separation by normal
distillation would then not be an option, so water or
steam is introduced into the distillation apparatus. By
adding water or steam, the boiling points of the
compounds are depressed, allowing them to
evaporate at lower temperatures, preferably below
the temperatures at which the deterioration of the
material becomes appreciable
Experiment IV: Distillation
STEAM DISTILLATION
Experiment IV: Distillation
STEAM DISTILLATION
Experiment IV: Distillation
STEAM DISTILLATION
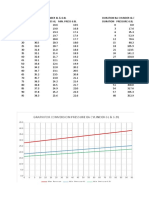
Volume Volume Weight Ratio
Xylene (mL) Water (mL)
First Fraction 0.5 1.3 0.33 : 1
Second -no data- -no data- -no data-
Fraction*
*the experiment was
unsuccessful
σ of xylene = 0.87 g/mL
σ of water = 1 g/mL
Experiment IV: Distillation
GUIDE QUESTIONS
Experiment IV: Distillation
Explain the differences of the distillation curves
between simple distillation and fractional
distillation.
Experiment IV: Distillation
SIMPLE vs FRACTIONAL DISTILLATION
Experiment IV: Distillation
In the separation of the ethanol from water
using fractional distillation, the distillate always
contains about 5% water. Explain.
Experiment IV: Distillation
t vs. % concentration of ethanol-water mixture
Experiment IV: Distillation
p vs. % concentration of ethanol-water mixture
Experiment IV: Distillation
t vs. % concentration of liquid mixture, which does not form azeotrope
Experiment IV: Distillation
Based on the phase diagram of 4(a), what is
the approximate composition of the ethanol-
water mixture, which begins to distill at 80°C?
Experiment IV: Distillation
At 95°C the vapor pressure of n-heptane is
684 mmHg and of n-octane is 303 mmHg.
Calculate the mole ratio and weight ratio of the
two components in a mixture of n-heptane and
n-octane, which begins to distill at 95°C at 650
mmHg.
Experiment IV: Distillation
P sol = 650 mmHg
684 x + 303 (1-x) = 650
684x + 303 – 303x = 650
x= 0.9107
1-x = 0.089
Mole ratio: 0.9107/0.089 = 10.23 (10.23 mols
of n-heptane for every 1 mol of n-octane)
Weight ratio: 91.25/10.17 = 8.98 (8.98 g of n-
heptane for every 1 g of n-octane)
Experiment IV: Distillation
Explain the difference in the values of
toluene/water weight ratios between the first
and second fractions.
Experiment IV: Distillation
Cite some important application of steam
distillation and vacuum distillation
Experiment IV: Distillation
You might also like
- Fundamental of MeterologyDocument447 pagesFundamental of Meterologypriya kumari100% (7)
- Simple and Fractional DistillationDocument18 pagesSimple and Fractional DistillationDaniel BuanNo ratings yet
- 15repeat Formation TesterDocument8 pages15repeat Formation TesterMark allenNo ratings yet
- Phase Separation PDFDocument34 pagesPhase Separation PDFdwaynesimon18No ratings yet
- Experiment 1: Distillation: Chem 200 LaboratoryDocument17 pagesExperiment 1: Distillation: Chem 200 LaboratoryLea PesiganNo ratings yet
- Fractional Distillattion: By: Aman Kumar To: Dr. ShivaniDocument16 pagesFractional Distillattion: By: Aman Kumar To: Dr. ShivanimohitNo ratings yet
- Boiler 2Document57 pagesBoiler 2azmisabran100% (1)
- CHM 207 Report 2Document8 pagesCHM 207 Report 2Salazar ZawawiNo ratings yet
- An Illustrated Parts BreakdownDocument2 pagesAn Illustrated Parts Breakdowngeofaxxxxxx100% (1)
- Pelton Wheel Lab ReportDocument7 pagesPelton Wheel Lab Reportws100% (1)
- API Training-Relieve Device (API 520)Document24 pagesAPI Training-Relieve Device (API 520)Dimas Eko Prasetyo100% (6)
- Simple DistillationDocument5 pagesSimple DistillationRyan Joseph GaholNo ratings yet
- Oisd STD 150Document27 pagesOisd STD 150Nanu PatelNo ratings yet
- Distillation 140815233428 Phpapp02Document18 pagesDistillation 140815233428 Phpapp02Vignesh MallyaNo ratings yet
- Technical Guide Steam Boilers (001 282)Document213 pagesTechnical Guide Steam Boilers (001 282)Sebastian Ignacio CavieresNo ratings yet
- Practice Makes Perfect in Chemistry: Organic ChemistryFrom EverandPractice Makes Perfect in Chemistry: Organic ChemistryRating: 3 out of 5 stars3/5 (1)
- Simple Distillation of VodkaDocument4 pagesSimple Distillation of VodkaKatrina TaracatacNo ratings yet
- Determination of Ethanol Content From Ginebra Gin by Fractional DistillationDocument5 pagesDetermination of Ethanol Content From Ginebra Gin by Fractional DistillationJeriz Marie GamboaNo ratings yet
- Distillation of Alcoholic BeveragesDocument11 pagesDistillation of Alcoholic BeveragesPaul Jhon EugenioNo ratings yet
- Exp 3Document9 pagesExp 3tamanranya234No ratings yet
- 6 - Simple DistillationDocument6 pages6 - Simple DistillationJade AsparinNo ratings yet
- Distillation Lab ReportDocument4 pagesDistillation Lab ReportLevison KasengaNo ratings yet
- Activity 1Document6 pagesActivity 1Junaid KhanNo ratings yet
- Simple and Fractional DistiillationDocument4 pagesSimple and Fractional DistiillationPaul James AlavaNo ratings yet
- Org Chem LabDocument7 pagesOrg Chem LabCriselda CarinoNo ratings yet
- Fractional DistillationDocument2 pagesFractional DistillationDianne Joy PascuaNo ratings yet
- EXPE5Document6 pagesEXPE5K-yanVehraaYomomaNo ratings yet
- Fractional DistillationDocument4 pagesFractional DistillationmymamforeverNo ratings yet
- Simple and Fractional DistillationDocument6 pagesSimple and Fractional Distillationralph_ong230% (1)
- Exp.1-Distillation, Simple and FractionalDocument18 pagesExp.1-Distillation, Simple and FractionalzazoNo ratings yet
- What Is Fractional DistillationDocument4 pagesWhat Is Fractional DistillationShubham WarseNo ratings yet
- Azeotropic Distillation of Toluene With MethanolDocument16 pagesAzeotropic Distillation of Toluene With MethanolNurtasha AtikahNo ratings yet
- Exp.1-Distillation Simple and FractionalDocument18 pagesExp.1-Distillation Simple and Fractionalsisi slayNo ratings yet
- RefineryDocument41 pagesRefineryawad awadNo ratings yet
- Speaking Practice Questions Answers PDFDocument85 pagesSpeaking Practice Questions Answers PDFkomal naeemNo ratings yet
- Separation and PurificationDocument41 pagesSeparation and PurificationAlea PrillyNo ratings yet
- Lab Report Org ChemDocument5 pagesLab Report Org ChemShella Mare CanizaresNo ratings yet
- PLab EditedDocument7 pagesPLab EditedChin RamosNo ratings yet
- Chem 31.1 DistillationDocument3 pagesChem 31.1 DistillationMonroe OrlinaNo ratings yet
- Purifying Alcoholic Beverage Using Simple and Fractional DistillationDocument4 pagesPurifying Alcoholic Beverage Using Simple and Fractional DistillationMaiah DinglasanNo ratings yet
- Chemistry 200 Lab: More Volatile, Lower Boiling PointDocument2 pagesChemistry 200 Lab: More Volatile, Lower Boiling PointPam GarciaNo ratings yet
- Chem Wrick Term 2Document18 pagesChem Wrick Term 2wrickm19No ratings yet
- Lab 1Document2 pagesLab 1ayilna_suhaila100% (4)
- Distillation of GinDocument6 pagesDistillation of GinJan Chester ChanNo ratings yet
- Organic Chemistry Different TestDocument5 pagesOrganic Chemistry Different TestNera AyonNo ratings yet
- Distillation: Separation and PurificationDocument80 pagesDistillation: Separation and PurificationAbdur RehmanNo ratings yet
- Separating Ethanol and Water by DistillationDocument2 pagesSeparating Ethanol and Water by DistillationerinNo ratings yet
- Fractional Distillation of Ginebra San Miguel GinDocument6 pagesFractional Distillation of Ginebra San Miguel GinHajime NakaegawaNo ratings yet
- Types of DistillationDocument6 pagesTypes of Distillationravi2007No ratings yet
- Simple Distillation (Petroleum)Document8 pagesSimple Distillation (Petroleum)hayder alaliNo ratings yet
- Lab ReportDocument10 pagesLab ReportKathleen De Vera BarrilNo ratings yet
- Chem Print1Document5 pagesChem Print1Donna VelascoNo ratings yet
- Lab 05Document6 pagesLab 05Areeba NaqviNo ratings yet
- Organic Chem Distillation Lab 5Document6 pagesOrganic Chem Distillation Lab 5api-281480695No ratings yet
- Simple DistillationDocument1 pageSimple DistillationjudieliciousNo ratings yet
- Making A Liquid Pure - DistillationDocument4 pagesMaking A Liquid Pure - DistillationSaad Bin JawaidNo ratings yet
- INDUSTRIAL CHEMISTRY Lecture # 2Document21 pagesINDUSTRIAL CHEMISTRY Lecture # 2Ali Abbas Aslam Ali Abbas AslamNo ratings yet
- Distillation Basics: Dharmsinh Desai UniversityDocument20 pagesDistillation Basics: Dharmsinh Desai UniversityGilles DakouriNo ratings yet
- Experiment 5 Distillation of EthanolDocument3 pagesExperiment 5 Distillation of EthanolMiru HiragiNo ratings yet
- Distillationbyankitayagnik 180430073733Document80 pagesDistillationbyankitayagnik 180430073733Syeda K Shah KazmiNo ratings yet
- (L6) - (JLD 3.0) - Solutions - 21th April.Document49 pages(L6) - (JLD 3.0) - Solutions - 21th April.manu sharmaNo ratings yet
- 16 Distillation NotesDocument6 pages16 Distillation Notesyown silvaNo ratings yet
- Distillation Is The Oldest Method Used For Separating Mixtures of LiquidsDocument4 pagesDistillation Is The Oldest Method Used For Separating Mixtures of LiquidsDanNo ratings yet
- Organic Chemistry Laboratory: Basra University College of Science and Technology Pharmacy DepartmentDocument11 pagesOrganic Chemistry Laboratory: Basra University College of Science and Technology Pharmacy DepartmentcrtgyhujikNo ratings yet
- Experiment Number 3 Distillation ObjectiveDocument5 pagesExperiment Number 3 Distillation ObjectiveChristine Mae C. AlmendralNo ratings yet
- Orange Oil ReportDocument14 pagesOrange Oil Reportapi-383568092No ratings yet
- 10 AzeotropeDocument24 pages10 AzeotropeXclipsionNo ratings yet
- Graph For Conversion Pressure Ba Cylinder 6L & 6.8LDocument7 pagesGraph For Conversion Pressure Ba Cylinder 6L & 6.8LFebriyanto Pandu PratamaNo ratings yet
- The Daily Evaporation Rate Test and Conversion Method For A NewDocument11 pagesThe Daily Evaporation Rate Test and Conversion Method For A NewMenen SimmonNo ratings yet
- ThaerRoomi WindTunnel PDFDocument15 pagesThaerRoomi WindTunnel PDFRajNo ratings yet
- HTTP WWW - Spiraxsarco.com Resources Steam-Engineering-tutorials Control-Applications Pressure-Contr NewDocument15 pagesHTTP WWW - Spiraxsarco.com Resources Steam-Engineering-tutorials Control-Applications Pressure-Contr NewPalash KayathwalNo ratings yet
- Manual Reparación Turbo GARRETDocument24 pagesManual Reparación Turbo GARRETFelipe Lepe MattaNo ratings yet
- Fluid Mechanics (BTME-301-18)Document13 pagesFluid Mechanics (BTME-301-18)Surjit Kumar GandhiNo ratings yet
- SPE - On The Quality of Data From Standard Gas-Condensate PVT ExperimentsDocument12 pagesSPE - On The Quality of Data From Standard Gas-Condensate PVT ExperimentsJamalNo ratings yet
- Module 3 Task 1Document3 pagesModule 3 Task 1Light HouseNo ratings yet
- Trabajo de ReadingDocument3 pagesTrabajo de ReadingPerez DavidNo ratings yet
- Mustang Series M116-52 or M6116-52 (Globe), M1116-52 or M61116-52 (Angle) Installation InstructionsDocument2 pagesMustang Series M116-52 or M6116-52 (Globe), M1116-52 or M61116-52 (Angle) Installation InstructionsWattsNo ratings yet
- Twin Vs Single Screw ComparisonDocument2 pagesTwin Vs Single Screw ComparisonRajeshNo ratings yet
- (SCAC Tropical) Cycle Logic (Eng) - 180914Document33 pages(SCAC Tropical) Cycle Logic (Eng) - 180914Tehnika SiingNo ratings yet
- Me 604 SampleDocument4 pagesMe 604 SampleannukiitNo ratings yet
- IDP-7 5991-7602EN DataSheet LRDocument2 pagesIDP-7 5991-7602EN DataSheet LRTinkerer GregNo ratings yet
- Compresor Leybold-Rw6000Document28 pagesCompresor Leybold-Rw6000emanuelNo ratings yet
- GATE Mechanical Engineering MCQ on Basic Concepts and Energy AnalysisDocument84 pagesGATE Mechanical Engineering MCQ on Basic Concepts and Energy AnalysisRajesh Verma75% (4)
- SGI White Paper Methane and CO2 Emissions WEB FINALDocument105 pagesSGI White Paper Methane and CO2 Emissions WEB FINALSomya Kumar SinghNo ratings yet
- ME 306-Hw04-2017-2Document5 pagesME 306-Hw04-2017-2Aswad Che RusminNo ratings yet
- 04 PVT SamplingDocument6 pages04 PVT SamplingMohammad Iqbal Mahamad AmirNo ratings yet
- MI-106 Tut ThermoDocument37 pagesMI-106 Tut ThermoDhananjayLekshmiNarayan100% (7)
- PC1260Document4 pagesPC1260hamadaNo ratings yet
- C 1 P DP: SORU: The Fugasity F (Atm) of A Gas at A Pressure P (Atm) and A Specified Temperature T Is GivenDocument8 pagesC 1 P DP: SORU: The Fugasity F (Atm) of A Gas at A Pressure P (Atm) and A Specified Temperature T Is GivenhhkkllNo ratings yet