Professional Documents
Culture Documents
Electrolyte Effects Activity or Concentration Not Mine
Uploaded by
Mark Cliffton BadlonCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Electrolyte Effects Activity or Concentration Not Mine
Uploaded by
Mark Cliffton BadlonCopyright:
Available Formats
Jess Sproul
Chapter 9 Notes
Chapter 9
Electrolyte Effects: Activity or
Concentration
9A-1: How Do Ionic Charges Affect
Equilibria?
The magnitude of the effect that the
electrolyte has is highly dependent on the
charge of the ions participating in the
equilibrium.
When only neutral species are involved in
the equilibrium, the electrolyte has no real
effect, no matter how concentrated.
9A-1: How Do Ionic Charges Affect
Equilibria? (cont.)
In the figure seen at the right,
the molar solubility of the doubly
charged ions in BaSO4 increases by
a factor of 2 in a solution of 0.02 M
KNO3 over a solution of pure water.
On the other hand, the molar
solubility of a solution of Ba(IO3)2 with
one singly and one doubly charged
ion only increases by a factor of 1.25
in a solution of 0.02 M KNO3 over a
solution of pure water. And finally,
the molar solubility of AgCl with two
singly charged ions only increases by
a factor of 1.2 in a solution of 0.02 M
KNO3 over a solution of pure water.
9A-2: What Is the Effect of Ionic
Strength on Equilibria? (cont.)
The ionic strength of a
solution of a strong
electrolyte consisting
solely of singly charged
ions is identical with its
total molar salt
concentration. But
when the ions are no
longer singly charged,
the ionic strength
increases.
Type
Electrolyte
Example
Ionic Strength*
1:1
NaCl
1:2
Ba(NO3)2,
Na2SO4
3c
1:3
Al(NO3)3,
Na3PO4
6c
2:2
MgSO4
*c = molarity of the salt
4c
9A-3: The Salt Effect
The
electrolyte effect results from the electrostatic
attractive and repulsive forces that exist between
the ions of an electrolyte and the ions involved in
an equilibrium. These forces cause each ion from
the dissociated reactant to be surrounded by a
sheath of solution that contains a slight excess of
electrolyte ions of opposite charge.
9A-3: The Salt Effect
The electrolyte effect results from the electrostatic attractive and repulsive forces
that exist between the ions of an electrolyte and the ions involved in an
equilibrium. These forces cause each ion from the dissociated reactant to be
surrounded by a sheath of solution that contains a slight excess of electrolyte
ions of opposite charge. These charged atmospheres lessen the charges on
the ions in equilibrium, the Ba2+ and SO42- in the figure, thus decreasing
their overall attraction to each other and increasing solubility.
9B: Activity Coefficients
The term activity, a, is used to account for the
effects of electrolytes on chemical equilibria.
The activity, or effective concentration, of species X
depends on the ionic strength of the medium and is
defined as
ax = x [X]
where ax is the activity of X, [X] is its molar
concentration, and x is a dimensionless quantity
called the activity coefficient.
The activity coefficient and the activity of X vary
with ionic strength.
9B-1: Properties of Activity
Coefficients
In dilute solutions, the activity coefficient for
a given species is independent of the nature
of the electrolyte and dependent only on
ionic strength.
For a given ionic strength, the activity
coefficient of an ion departs farther from
unity as the charge carried by the species
increases.
At any given ionic strength, the activity
coefficients of ions of the same charge are
approximately equal.
The activity coefficient of a given ion
describes its effective behavior in all
equilibria in which it participates.
9B-1: Properties of Activity
Coefficients (cont.)
9B-2) The Debye-Hckel Equation
The Debye-Hckel equation takes the ionic
atmosphere model and derives a theoretical
expression that permits the calculation of
activity coefficients of ions from their charge
and their average size.
9B-3: Equilibrium Calculations with
Activity Coefficients
Equilibrium
calculations with
activities yield
values that are
better in agreement
with experimental
results than those
obtained with molar
concentrations.
9B-4: Omitting Activity Coefficients
in Equilibrium Calculations
We shall ordinarily neglect activity coefficients
and simply use molar concentrations in
applications of the equilibrium law. This
simplifies calculations and decreases
necessary data. For most purposes, the error
introduced by this method is minimal.
Significant discrepancies occur when the ionic
strength is larger than 0.01 or when the ions
involved have multiple charges.
With dilute solutions of nonelectrolytes or of simply
charged ions, the use of concentrations in a masslaw calculation often provides reasonably accurate
results.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Nat Sci July 2015Document15 pagesNat Sci July 2015Mark Cliffton BadlonNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Introduction To Infrared SpectrosDocument18 pagesIntroduction To Infrared SpectrosMark Cliffton BadlonNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Period (Year Started - Year Ended) Field University/ School Scholarship (If Applicable) RemarksDocument3 pagesPeriod (Year Started - Year Ended) Field University/ School Scholarship (If Applicable) RemarksMark Cliffton BadlonNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Section 1-Verbal Ability: 30 Questions-30 MinutesDocument10 pagesSection 1-Verbal Ability: 30 Questions-30 MinutesApocalypto StatumNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Introduction To Spectroscopic Methods: Instrumental AnalysisDocument27 pagesIntroduction To Spectroscopic Methods: Instrumental AnalysisMark Cliffton BadlonNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Introduction To UltravioletVisible Molecular Absorption SpectrometryDocument19 pagesIntroduction To UltravioletVisible Molecular Absorption SpectrometryMark Cliffton BadlonNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Introduction To UltravioletVisible Molecular Absorption SpectrometryDocument19 pagesIntroduction To UltravioletVisible Molecular Absorption SpectrometryMark Cliffton BadlonNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Introduction & Applications of Infrared SpectrometryDocument20 pagesIntroduction & Applications of Infrared SpectrometryMark Cliffton BadlonNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- High Performance Liquid ChromatographyDocument22 pagesHigh Performance Liquid ChromatographyDeden Aldila ZulkhidaNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- How Equilibrium Calculations Can Be Applied To Complex SystemsNot MineDocument16 pagesHow Equilibrium Calculations Can Be Applied To Complex SystemsNot MineMark Cliffton BadlonNo ratings yet
- How Equilibrium Calculations Can Be Applied To Complex Systems Not MineDocument11 pagesHow Equilibrium Calculations Can Be Applied To Complex Systems Not MineMark Cliffton BadlonNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Infrared SpectrometryDocument18 pagesInfrared SpectrometryMark Cliffton BadlonNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- High Performance Liquid ChromatographyDocument17 pagesHigh Performance Liquid ChromatographyOsama BakheetNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- High Performance Liquid ChromatographyDocument17 pagesHigh Performance Liquid ChromatographyOsama BakheetNo ratings yet
- FTIR SOP Not MineDocument1 pageFTIR SOP Not MineMark Cliffton BadlonNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Not MineDocument3 pagesEvaluation of Analytical Parameters in Atomic Absorption Spectroscopy Not MineMark Cliffton BadlonNo ratings yet
- Gas Chromatography Not MineDocument14 pagesGas Chromatography Not MineMark Cliffton BadlonNo ratings yet
- GC1 Not MineDocument18 pagesGC1 Not MineMark Cliffton BadlonNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Gas Chromatography Not MineDocument28 pagesGas Chromatography Not MineMark Cliffton BadlonNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Evaluation of A Specific Ion Electrode Not MineDocument7 pagesEvaluation of A Specific Ion Electrode Not MineMark Cliffton BadlonNo ratings yet
- Electroanalytical Chemistry (Nadya) Ver.2 Not MineDocument37 pagesElectroanalytical Chemistry (Nadya) Ver.2 Not MineMark Cliffton BadlonNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Electrolyte Effects Activity or Concentration Not MineDocument14 pagesElectrolyte Effects Activity or Concentration Not MineMark Cliffton BadlonNo ratings yet
- Electroanalytical Chemistry Not MineDocument37 pagesElectroanalytical Chemistry Not MineMark Cliffton BadlonNo ratings yet
- Electrical Components and Circuits Not MineDocument22 pagesElectrical Components and Circuits Not MineMark Cliffton BadlonNo ratings yet
- Digital Electronics, MicroprocessorsDocument21 pagesDigital Electronics, Microprocessorsjeetu1jeetu1No ratings yet
- Electroanalytical Chemistry (1) Not MineDocument23 pagesElectroanalytical Chemistry (1) Not MineMark Cliffton BadlonNo ratings yet
- Electrical Components and Circuits Not MineDocument21 pagesElectrical Components and Circuits Not MineMark Cliffton BadlonNo ratings yet
- Digital Electronics Microprocessors and Computers Not MineDocument11 pagesDigital Electronics Microprocessors and Computers Not MineMark Cliffton BadlonNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Development of The Periodic Table Chem 111 Not MineDocument7 pagesDevelopment of The Periodic Table Chem 111 Not MineMark Cliffton BadlonNo ratings yet
- Chemistry I - Chapter 19 Chemistry I HD - Chapter 16 ICP - Chapter 23Document74 pagesChemistry I - Chapter 19 Chemistry I HD - Chapter 16 ICP - Chapter 23Brandeice BarrettNo ratings yet
- 11 Chemistry Solved Questions Chapter 7Document7 pages11 Chemistry Solved Questions Chapter 7Mohd UvaisNo ratings yet
- Exercise 2 For Solution ThermodynamicsDocument4 pagesExercise 2 For Solution ThermodynamicsShane MillenaNo ratings yet
- Kinetics and Mechanism of Thermal Decomposition of GaNDocument7 pagesKinetics and Mechanism of Thermal Decomposition of GaNjhlinjNo ratings yet
- SM 11 Chemistry Eng 201617Document185 pagesSM 11 Chemistry Eng 201617Anonymous 9uu04el100% (1)
- Course - Planner - Praveen-III (XIII)Document2 pagesCourse - Planner - Praveen-III (XIII)Manju Mittal67% (3)
- WCH06 01 Que 20160517Document24 pagesWCH06 01 Que 20160517miran abbassNo ratings yet
- Questions Paper 2 CHP 19Document5 pagesQuestions Paper 2 CHP 19FIKRIYE ONDEROLNo ratings yet
- The Adverse Effects of Le Châtelier's Principle On Teacher Understanding of Chemical EquilibriumDocument5 pagesThe Adverse Effects of Le Châtelier's Principle On Teacher Understanding of Chemical EquilibriumCharlie GreenNo ratings yet
- Chemistry Total Mark: 100 Appendix A' (Outlines of Tests)Document8 pagesChemistry Total Mark: 100 Appendix A' (Outlines of Tests)Iqra AfzalNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Lenard Jones PotentialDocument3 pagesLenard Jones PotentialKailasham RamalingamNo ratings yet
- Chem SolutionsDocument39 pagesChem SolutionsKian Justin LagritoNo ratings yet
- Performance of A Small Scale Haber ProcessDocument9 pagesPerformance of A Small Scale Haber ProcessGuilherme SouzaNo ratings yet
- CHE502 - Reaction Engineering 1 AssignmeDocument22 pagesCHE502 - Reaction Engineering 1 AssignmeShilpa KodolikarNo ratings yet
- Calculation of Equilibrium in The System Metal - Slag During Steelmaking in Electric Arc FurnaceDocument6 pagesCalculation of Equilibrium in The System Metal - Slag During Steelmaking in Electric Arc FurnaceWaleed YossefNo ratings yet
- Chemical Equilibruim - With Solutions-Review 2013Document15 pagesChemical Equilibruim - With Solutions-Review 2013ShreyaNo ratings yet
- 07 17 2014 IChO46 Preparatory SolutionsDocument82 pages07 17 2014 IChO46 Preparatory SolutionsPhuc NguyenNo ratings yet
- 11 Chemical EquilibriumDocument43 pages11 Chemical EquilibriumRishma GuptaNo ratings yet
- Chapter10 IntroDocument15 pagesChapter10 IntroAmirul AfiqNo ratings yet
- Torsion 6th ChapterDocument13 pagesTorsion 6th ChapterakshatbhargavaNo ratings yet
- Lesson Plan: A. Learning ObjectivesDocument4 pagesLesson Plan: A. Learning ObjectivesNazla Qonita PonotNo ratings yet
- Elimination of Bitter, Disgusting Tastes of Drugs and Foods by CyclodextrinsDocument11 pagesElimination of Bitter, Disgusting Tastes of Drugs and Foods by CyclodextrinswisievekNo ratings yet
- III Chemical Sciences A-02-03: Name & Signature of InvigilatorDocument15 pagesIII Chemical Sciences A-02-03: Name & Signature of InvigilatorJaju VasuNo ratings yet
- Oct/june 20 Unit 6 Chemistry Edexcel IAL MarkschemeDocument22 pagesOct/june 20 Unit 6 Chemistry Edexcel IAL MarkschemeZubanaNo ratings yet
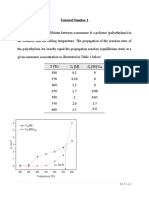
- Tutorial Number 1jjkDocument4 pagesTutorial Number 1jjkSaif JassimNo ratings yet
- Chapter 22 IsmDocument14 pagesChapter 22 Ismvpetro250No ratings yet
- Physical Chemistry 2Document288 pagesPhysical Chemistry 2Islombek TurgunboevNo ratings yet
- ACS Practice Test 1Document10 pagesACS Practice Test 1drwams100% (2)
- Le Chatelier LabDocument3 pagesLe Chatelier LabDeep ManNo ratings yet
- Arizona, Utah & New Mexico: A Guide to the State & National ParksFrom EverandArizona, Utah & New Mexico: A Guide to the State & National ParksRating: 4 out of 5 stars4/5 (1)
- South Central Alaska a Guide to the Hiking & Canoeing Trails ExcerptFrom EverandSouth Central Alaska a Guide to the Hiking & Canoeing Trails ExcerptRating: 5 out of 5 stars5/5 (1)