Professional Documents
Culture Documents
S1.2 PPT DR - Taufik Sungkar
Uploaded by
MuhammadIkhsanFadillah0 ratings0% found this document useful (0 votes)
20 views32 pagesOriginal Title
S1.2 PPT dr.Taufik Sungkar.ppt
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
20 views32 pagesS1.2 PPT DR - Taufik Sungkar
Uploaded by
MuhammadIkhsanFadillahCopyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 32
Management of Hepatitis C Infection in the
Era of Direct-Acting Antiviral Therapy
Taufik Sungkar
Division of Gastroenterology and Hepatology, Faculty of Medicine,
University of Sumatera Utara
Introduction
• To date, hepatitis C viral infection remains to be a
global health challenge. Nearly 180 millions of people
were infected worldwide, with reported debilitating
complications as high as 25% including cirrhosis and
hepatocellular carcinoma.
• For many years, standard therapy consists of pegylated
interferon α (pegIFNα) with additional ribavirin (RBV).
In terms of SVR, this early era therapy showed poor rates
and varied greatly among genomes.
• Newer regimens have been developed to improve cure
rate, increase tolerability thus hopefully prevent
complications.
• In the current DAA era, recent recommendations
approve interferon-free, some are ribavirin-free, DAAs
combination, which mainly suggested according to its
genotypes.
Prevalence
N.J Burstow et al. International Journal of General Medicine 2017:10 39-52
N.J Burstow et al. International Journal of General Medicine 2017:10 39-52
Evolution of HCV therapy
Previous Interferon-Ribavirin Therapy
• Up until 2011, the combination of pegylated interferon α
(pegIFNα) with ribavirin (RBV) has been used as a
standard regimen for HCV, that consisted of 24-48
weeks of treatment duration.
• Treatment outcome varied greatly among genomes.
Despite being the most common, GT1 remains the most
difficult to control, with SVR of only 40% following
completion of 48 weeks standard therapy
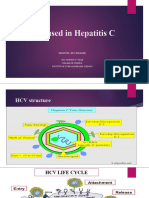
Direct-Acting Antiviral (DAA)
• Hepatitis C life cycle and the targets of direct-acting
antiviral agents.
Direct-acting antivirals for HCV
T. Asselah et al. Liver Int. 2016; 36 (Suppl. S1): 47–57
Development of Direct-Acting Antiviral (DAA)
Table 1. Directly acting antivirals and sites of action
N.J Burstow et al. International Journal of General Medicine 2017:10 39-52
• First generation NS3/4A PIs include telaprevir (TVR) and
boceprevir (BOC) that had been approved in 2011 for
treating GT1 infection.
• This approach greatly improve SVR of up to 75%,
however observed effective in GT1 only. Other than
exclusively potent for GT1, side effects were also common
that includes anemia, rash, and fatigue contributing to
high drop off rate.
• Consequently, both first-generation NS3/4A inhibitor
Telaprevir and Boceprevir was withdrawn from the
market since 2014
Newer Agents of DAAs
Table 2. Interferon-free treatment regimens for chronic hepatitis C
according to cirrhosis and treatment status, as recommended by the
European Association for the study of the liver and AASLD.
N.J Burstow et al. International Journal of General Medicine 2017:10 39-52
N.J Burstow et al. International Journal of General Medicine 2017:10 39-52
Spesial Populations
• DAAs therapy for chronic hepatitis C among patients in
the liver transplant setting
o Since DAA treatment seems to substantially improve liver
function in at least some patients with decompensated cirrhosis,
there may be patients who can be spared from LT.
o A recent retrospective European study evaluated 103 HCV-
infected patients on the liver transplant waiting list because of
decompensated cirrhosis who received DAA therapy.
o At 6 months after treatment initiation 16% of patients was
inactivated (placed ‘on hold’ due to clinical improvement but
not yet removed from the waiting list), but no one was delisted.
Table 3. Antiviral treatment regimens in liver transplant
recipients
• Hepatitis C viral infection in chronic kidney disease
o HCV in CKD population is prevalent. Prevalence of ESRD with
HCV varied from 1% to 70% according to its country of origin,
with incidence rate of 0.97 to 4.44 in dialysis patients.
o Current management of HCV in patients with renal impairment
includes combination of DAAs, often interferon-free.
o According to EASL recommendation in 2016, patients with mild
to moderately impared renal functions (eGFR ≥ 30 ml/min/1.73
m2) requires no dose adjustment to several regimens, including
SOF/RBV, SOF/LDV, SOF/VEL, RTV-PTV/OMB/DSV, GZR/EBR,
SOF/DCV or SOF/SMV. Hence to be treated in a similar fashion as
to non-CKD cases .
o With a number of DAAs being excreted and accumulated in
kidneys special considerations need to be made in severe renal
impairment. In patients with eGFR <30 ml/min/1.73 m2)
,including stage 4 and 5 CKD or hemodialysis patients, the use of
the renally excreted sofosbuvir is not recommended.
o The C-SURFER trial also studied sofosbuvir-free, grazoprevir and
elbasvir regimen for 12 weeks, which showed similarly high 94%
SVR.
Table 4. Main studies evaluating interferon-free regimen in
HCV patients with chronic kidney disease
Patrice Cacoub et al. Journal of Hepatology 2016 vol. 65 j S82–S94
Patrice Cacoub et al. Journal of Hepatology 2016 vol. 65 j S82–S94
HCV and HBV coinfection
• The goal of therapy in HBV and HCV coinfection is to
eradicate HCV infection and inhibit HBV replication.
• Evaluation of liver disease progression, predominance of
one virus over another, and comorbidities are essential
for optimal antiviral regimens.
• For patients with active hepatitis C, the same regimens
following the same rules as for monoinfected patients
should be applied based on AASLD and EASL
recommendations
• For patients with active hepatitis B before, during or
after HCV clearance or with established cirrhosis,
nucleoside or nucleotide analog (NA), tenofovir or
entecavir is indicated.
• Prior initiating DAA-based treatment for hepatitis C,
patients should be tested for HBs antigen, anti-HBc
antibodies and anti-HBs antibodies. If HBs antigen is
present or if HBV DNA is detectable in HBs antigen-
negative, anti-HBc antibody-positive patients (“occult”
hepatitis B), concurrent HBV nucleoside/nucleotide
analog therapy is indicated.
HCV and HIV coinfection
• Patients with HCV and HIV coinfection are treated
according to genotype and prior treatment status as
follows:
(1) Genotype 1a, treatment-naïve patients may be treated
with any of the following regimen:
a. Sofosbuvir/ledipasvir for 12 weeks without ribavirin
b. Sofosbuvir/velpatasvir for 12 weeks without ribavirin
c. Ombitasvir/paritaprevir/ritonavir and dasabuvir for 12 weeks
with ribavirin
d. Grazoprevir/elbasvir for 12 weeks without ribavirin if HCV
RNA=800,000 IU/ml or 16 weeks with ribavirin if HCV RNA
>800,000 IU/ml
e. Sofosbuvir/daclatasvir for 12 weeks without ribavirin
• Genotype 1a, treatment-experienced patients may be
treated with any of the following regimen:
1. Sofosbuvir/ledipasvir for 12 weeks with ribavirin or 24 weeks
without ribavirin
2. Sofosbuvir/velpatasvir for 12 weeks without ribavirin
3. Ombitasvir/paritaprevir/ritonavir and dasabuvir for 12 weeks
with ribavirin
4. Grazoprevir/elbasvir for 12 weeks without ribavirin if HCV
RNA=800,000 IU/ml or 16 weeks with ribavirin if HCV RNA
>800,000 IU/ml
5. Sofosbuvir/daclatasvir for 12 weeks with ribavirin or 24 weeks
without ribavirin
(2) Genotype 1b, treatment-naïve and treatment-
experienced patients may be treated with any of the
following regimen:
a. Sofosbuvir/ledipasvir for 12 weeks without ribavirin
b. Sofosbuvir/velpatasvir for 12 weeks without ribavirin
c. Ombitasvir/paritaprevir/ritonavir and dasabuvir for 12
weeks with ribavirin
d. Grazoprevir/elbasvir for 12 weeks without ribavirin
e. Sofosbuvir/daclatasvir for 12 weeks without ribavirin
• Genotype 2, treatment-naïve and treatment-
experienced patients may be treated with any of the
following regimen:
a. Sofosbuvir/velpatasvir for 12 weeks without ribavirin
b. Sofosbuvir/daclatasvir for 12 weeks without ribavirin
• Genotype 3, treatment-naïve and treatment-
experienced patients may be treated with any of the
following regimen:
a.Sofosbuvir/velpatasvir for 12 weeks with ribavirin or
24 weeks without ribavirin
b.Sofosbuvir/daclatasvir for 12 weeks with ribavirin
• Genotype 4 treatment-naïve patients may be treated
with any of the following regimen:
a. Sofosbuvir/ledipasvir for 12 weeks without ribavirin
b. Sofosbuvir/velpatasvir for 12 weeks without ribavirin
c. Ombitasvir/paritaprevir/ritonavir with ribavirin for 12 weeks
d. Grazoprevir/elbasvir for 12 weeks without ribavirin
e. Sofosbuvir/daclatasvir for 12 weeks without ribavirin
f. Sofosbuvir and simeprevir for 12 weeks without ribavirin
• Genotype 4 treatment-experienced patients may be
treated with any of the following regimen:
a. Sofosbuvir/ledipasvir for 12 weeks with ribavirin and 24 weeks with ribavirin
b. Sofosbuvir/velpatasvir for 12 weeks without ribavirin
c. Ombitasvir/paritaprevir/ritonavir with ribavirin for 12 weeks
d. Grazoprevir/elbasvir for 12 weeks without ribavirin if HCV RNA =800,000 or
16 weeks with ribavirin if HCV RNA >800,000 IU/ml
e. Sofosbuvir/daclatasvir for 12 weeks with ribavirin or 24 weeks without
ribavirin
f. Sofosbuvir and simeprevir for 12 weeks with ribavirin or 24 weeks without
ribavirin
Conclusions
• The last few years has shown rapidly growing discoveries
in hepatitis C treatment. Previous interferon-ribavirin-
first generation DAAs has not been a favourable options,
leading to further investigation of DAAs combinations.
• DAAs combinations has shown great SVR, some even
achieved complete SVR.
You might also like
- Hepatitis C Virus-Host Interactions and Therapeutics: Current Insights and Future PerspectivesFrom EverandHepatitis C Virus-Host Interactions and Therapeutics: Current Insights and Future PerspectivesNo ratings yet
- Final-HCV Guidelines Treatment 2018Document37 pagesFinal-HCV Guidelines Treatment 2018Joriza TamayoNo ratings yet
- Top Trials in Gastroenterology & HepatologyFrom EverandTop Trials in Gastroenterology & HepatologyRating: 4.5 out of 5 stars4.5/5 (7)
- Sofosbuvir and Velpatasvir For HCV in Patients With Decompensated CirrhosisDocument12 pagesSofosbuvir and Velpatasvir For HCV in Patients With Decompensated CirrhosisanaNo ratings yet
- AASLD Recommendations For HCVDocument45 pagesAASLD Recommendations For HCVRamy ElbarodyNo ratings yet
- Treatment Ciroza HepaticaDocument88 pagesTreatment Ciroza Hepaticaanon_966944544100% (1)
- Choice of Daas For HCV Genotype 2: Eyond GuidelinesDocument32 pagesChoice of Daas For HCV Genotype 2: Eyond GuidelinesLiao Jian-QiangNo ratings yet
- Journal Club Presentation - Hepatitis CDocument48 pagesJournal Club Presentation - Hepatitis Cadilah fazliNo ratings yet
- Pharmacological Management of Hepatitis CDocument62 pagesPharmacological Management of Hepatitis CWei HangNo ratings yet
- HCV Unique PopulationsDocument66 pagesHCV Unique PopulationsManzoorAlamKhattakNo ratings yet
- Drugs Used in Hep CDocument57 pagesDrugs Used in Hep CRiny KhurshidNo ratings yet
- Treatment HCV Genotype 3 PDFDocument27 pagesTreatment HCV Genotype 3 PDFHanifa RamadhaniNo ratings yet
- Efficacy and Safety of Daclatasvir Plus Sofosbuvir For Treatment-Naïve and Treatment-Experienced Egyptian Patients With Hepatitis C Virus InfectionDocument13 pagesEfficacy and Safety of Daclatasvir Plus Sofosbuvir For Treatment-Naïve and Treatment-Experienced Egyptian Patients With Hepatitis C Virus InfectionIslam NasrNo ratings yet
- Toksisitas Hati Terkait Dengan Sofosbuvir, Inhibitor NS5A: Laporan KasusDocument9 pagesToksisitas Hati Terkait Dengan Sofosbuvir, Inhibitor NS5A: Laporan KasusAldy WhisnuNo ratings yet
- Hepatitis B Virus and Hepatitis C Virus Co-Infection With HIVDocument54 pagesHepatitis B Virus and Hepatitis C Virus Co-Infection With HIVShree Narayan YadavNo ratings yet
- Diagnosis &treatment of Hep CDocument18 pagesDiagnosis &treatment of Hep CMushtaq AhmadNo ratings yet
- Awareness On HepatitisDocument52 pagesAwareness On HepatitisMizanur RahmanNo ratings yet
- Toksisitas Hati Terkait Dengan Sofosbuvir, Inhibitor NS5A: Laporan KasusDocument9 pagesToksisitas Hati Terkait Dengan Sofosbuvir, Inhibitor NS5A: Laporan KasusAldy WhisnuNo ratings yet
- Treatment of Liver CirrhosisDocument85 pagesTreatment of Liver CirrhosisPetru Boaghi100% (1)
- Update On MGT of Hepatitis B and CDocument57 pagesUpdate On MGT of Hepatitis B and Cadamu mohammadNo ratings yet
- Success of (NS5BNS5A Inhibitors) SofosbuvirVelpatasvir in Management Hepatitis C Studied Tertiary Care Healthcare in Rawalpindi, PakistanDocument6 pagesSuccess of (NS5BNS5A Inhibitors) SofosbuvirVelpatasvir in Management Hepatitis C Studied Tertiary Care Healthcare in Rawalpindi, PakistanInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Treatment of HCV in Persons With HIV CoinfectionDocument17 pagesTreatment of HCV in Persons With HIV CoinfectionSinziana OgrutanNo ratings yet
- Bioscientific Review (BSR)Document9 pagesBioscientific Review (BSR)UMT JournalsNo ratings yet
- Management of Chronic Hepatitis C in AdultsDocument8 pagesManagement of Chronic Hepatitis C in AdultsJye yiNo ratings yet
- 117 Full PDFDocument13 pages117 Full PDFPang-Hom EstellaNo ratings yet
- Recommendations For Testing, Managing, and Treating Hepatitis C - InITIAL TREATMENT of HCV INFECTIONDocument20 pagesRecommendations For Testing, Managing, and Treating Hepatitis C - InITIAL TREATMENT of HCV INFECTIONFauzan AprnNo ratings yet
- HCV Cure For Everyone or Which Challenges Remain?: ReviewDocument4 pagesHCV Cure For Everyone or Which Challenges Remain?: Reviewapi-206282033No ratings yet
- South African Hepatitis C Management Guidelines 2010Document18 pagesSouth African Hepatitis C Management Guidelines 2010RatnaSuryatiNo ratings yet
- ContentServer - Asp 47Document8 pagesContentServer - Asp 47TataNo ratings yet
- C. 48-Year-Old Man Who Has Sex With Men: AlasanDocument12 pagesC. 48-Year-Old Man Who Has Sex With Men: AlasanAjeng WidyastutiNo ratings yet
- Toksisitas Hati Terkait Dengan Sofosbuvir, Inhibitor NS5A: Laporan KasusDocument6 pagesToksisitas Hati Terkait Dengan Sofosbuvir, Inhibitor NS5A: Laporan KasusAldy WhisnuNo ratings yet
- Hepatitis C ManagementDocument86 pagesHepatitis C ManagementArif SamoonNo ratings yet
- Treatment Management GuideDocument41 pagesTreatment Management GuidemarcelinaiNo ratings yet
- Viral HepatitisDocument25 pagesViral HepatitisRiaanCombrinck100% (1)
- Elbasvir + Grazoprevir - RecommendationDocument4 pagesElbasvir + Grazoprevir - RecommendationPaul Francis UyNo ratings yet
- Acute & Chronic HepatitisDocument65 pagesAcute & Chronic HepatitisMahmoud AjinehNo ratings yet
- Treatment Ciroza HepaticaDocument85 pagesTreatment Ciroza HepaticaMarina TrofimciucNo ratings yet
- Treatment of Liver Cirrhosis: Berliba Elina, MDDocument92 pagesTreatment of Liver Cirrhosis: Berliba Elina, MDRadu TuguiNo ratings yet
- HepatitisDocument5 pagesHepatitisnurhasanah2112No ratings yet
- Treatment of Rheumatoid Arthritis in Patients With Concomitant Chronic Hepatitis C InfectionDocument24 pagesTreatment of Rheumatoid Arthritis in Patients With Concomitant Chronic Hepatitis C InfectionMuhammad Farras Razin PerdanaNo ratings yet
- HCV GuidelinesDocument25 pagesHCV GuidelinesBilal NafeesNo ratings yet
- Hepatits CDocument2 pagesHepatits CFoxtrot NursingNo ratings yet
- Bliss LNDocument37 pagesBliss LNNeelam Devi MaraviNo ratings yet
- HepC Treatment 2015Document38 pagesHepC Treatment 2015Claudia IrinaNo ratings yet
- Antiretroviral TreatmentDocument32 pagesAntiretroviral TreatmentStanley Tatenda MukonoNo ratings yet
- Adefovir Dipivoxil 10mg (Hepsera)Document15 pagesAdefovir Dipivoxil 10mg (Hepsera)ddandan_2No ratings yet
- Chronic Hepatitis B Infection in ChildrenDocument4 pagesChronic Hepatitis B Infection in ChildrenSara KhanNo ratings yet
- 5e2c2028c2186 SofosbuvirDocument8 pages5e2c2028c2186 SofosbuvirAna Maria RusuNo ratings yet
- Lamivudine For Patients With Chronic Hepatitis B and Advanced Liver DiseaseDocument11 pagesLamivudine For Patients With Chronic Hepatitis B and Advanced Liver DiseaseRia DeviNo ratings yet
- 2 AnemiaDocument13 pages2 AnemiasalwaNo ratings yet
- Sofosbuvir For Previously Untreated Chronic Hepatitis C InfectionDocument10 pagesSofosbuvir For Previously Untreated Chronic Hepatitis C InfectionSitta Grewo LiandarNo ratings yet
- Hepatitis BDocument48 pagesHepatitis BHari Suthan TNo ratings yet
- Hep B RekomendasiDocument5 pagesHep B RekomendasiKevin JawanNo ratings yet
- Viral Hepatitis 2015Document54 pagesViral Hepatitis 2015Abdulziz Al-jedaieNo ratings yet
- Entecavir Combined With Short-Term Hepatitis B Immunoglobulin in Preventing Hepatitis B Virus Recurrence in Liver Transplant RecDocument1 pageEntecavir Combined With Short-Term Hepatitis B Immunoglobulin in Preventing Hepatitis B Virus Recurrence in Liver Transplant RecAliabdulghaniNo ratings yet
- DVT & Pe (R)Document10 pagesDVT & Pe (R)may myatNo ratings yet
- Garuda 762976Document5 pagesGaruda 762976bimaNo ratings yet
- Sofosbuvir For Previously Untreated Chronic Hepatitis C InfectionDocument23 pagesSofosbuvir For Previously Untreated Chronic Hepatitis C InfectionalfianfirdausNo ratings yet
- Effect of FoodDocument25 pagesEffect of FoodpathuriNo ratings yet
- Seden K, Et Al. Drug Inter at Ions of ARV and Directly Acting AntiviralsDocument7 pagesSeden K, Et Al. Drug Inter at Ions of ARV and Directly Acting AntiviralsErica LeeNo ratings yet
- Sistik FibrosisDocument5 pagesSistik FibrosisMuhammadIkhsanFadillahNo ratings yet
- S5.1Presentation LeptospiraDocument19 pagesS5.1Presentation LeptospiraMuhammadIkhsanFadillahNo ratings yet
- Tuberculous Pleural EffusionDocument26 pagesTuberculous Pleural EffusionMuhammadIkhsanFadillahNo ratings yet
- Amnoitic FluidDocument20 pagesAmnoitic FluidMuhammadIkhsanFadillahNo ratings yet
- GoutDocument6 pagesGoutMuhammadIkhsanFadillahNo ratings yet
- GoutDocument6 pagesGoutMuhammadIkhsanFadillahNo ratings yet
- Pediatric 6th Year 2016Document30 pagesPediatric 6th Year 2016motasem alsharifNo ratings yet
- World Congress of Internal Medicine (WCIM) 2014 Poster PresentationDocument34 pagesWorld Congress of Internal Medicine (WCIM) 2014 Poster PresentationTenri AshariNo ratings yet
- HepatocytesDocument38 pagesHepatocytesapi-327824087No ratings yet
- DRUG NAME: Rituximab: Synonym (S) : Common Trade Name (S) : ClassificationDocument12 pagesDRUG NAME: Rituximab: Synonym (S) : Common Trade Name (S) : ClassificationpmuftiaNo ratings yet
- Liver CancerDocument44 pagesLiver CancerJoyce Ann CumlatNo ratings yet
- Project Report On Hepatitis VirusDocument85 pagesProject Report On Hepatitis VirusBrijesh Singh Yadav100% (1)
- 1gastrointestinal System Disorders NCLEX Practice QuizDocument8 pages1gastrointestinal System Disorders NCLEX Practice QuizmyNo ratings yet
- Case Study ExtraDocument5 pagesCase Study ExtraNikhilesh PrasadNo ratings yet
- Twinrix Product MonographDocument27 pagesTwinrix Product Monographandy175No ratings yet
- Alcoholic Liver DiseaseDocument70 pagesAlcoholic Liver Diseaseaannaass nNo ratings yet
- 108pages On HEALTHDocument108 pages108pages On HEALTHsorin100% (2)
- Case Study 1 - Ulcerative ColitisDocument17 pagesCase Study 1 - Ulcerative ColitisJossua RyanNo ratings yet
- Graphic Guide Diseases PDFDocument382 pagesGraphic Guide Diseases PDFRafael100% (3)
- WHO 9789241565639-EngDocument476 pagesWHO 9789241565639-Engsofiabloem100% (1)
- Notes On Liver Colon CancerDocument16 pagesNotes On Liver Colon CancerElleNo ratings yet
- Lallo. Broiler Duck Production ManualDocument82 pagesLallo. Broiler Duck Production ManualJTheron5850% (2)
- Bmri2019 3951574Document10 pagesBmri2019 3951574Anonymous 1EQutBNo ratings yet
- Pediatric Liver TransplantationDocument30 pagesPediatric Liver TransplantationMadhu Sinha100% (1)
- Guidance For Visiting Medical Students and Overseas Elective Medical Students 1Document3 pagesGuidance For Visiting Medical Students and Overseas Elective Medical Students 1sdfNo ratings yet
- MIMS Doctor July 2017 MYDocument68 pagesMIMS Doctor July 2017 MYChloe LeNo ratings yet
- MedScholar MDCN Lesson Timetable. June, 2024Document10 pagesMedScholar MDCN Lesson Timetable. June, 202495bc5fgd7jNo ratings yet
- Evidence Based-How To Ask An Answerable QuestionsDocument62 pagesEvidence Based-How To Ask An Answerable QuestionsFeniNo ratings yet
- Clinical Microbiology: Sigma Metrics For Assessing Accuracy of Molecular TestingDocument8 pagesClinical Microbiology: Sigma Metrics For Assessing Accuracy of Molecular Testingwolfsblut33No ratings yet
- Immunodot Torch Test 410346Document11 pagesImmunodot Torch Test 410346Lucian MihuNo ratings yet
- Sulfasalazine Toxic Reactions. Hepatitis, Fever, and Skin Rash With Hypocomplementemia and Immune ComplexesDocument2 pagesSulfasalazine Toxic Reactions. Hepatitis, Fever, and Skin Rash With Hypocomplementemia and Immune ComplexesChistian LassoNo ratings yet
- MR - Mohankumar ReportDocument3 pagesMR - Mohankumar ReportJayaprabhu Prabhu0% (1)
- Chronic Liver Disease and Silymarin: A Biochemical and Clinical ReviewDocument5 pagesChronic Liver Disease and Silymarin: A Biochemical and Clinical ReviewEdwin ThomasNo ratings yet
- Pathology IcsmDocument84 pagesPathology IcsmAlice TangNo ratings yet
- Health Strategy: PTI's Health VisionDocument67 pagesHealth Strategy: PTI's Health VisionPTI Official100% (14)
- Chronic Conditions QDocument47 pagesChronic Conditions QahmadranauiNo ratings yet